4.2: Free Radical Reactions
- Page ID
- 416446
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Understand free radical reaction mechanisms, including elementary reactions of its three main steps: initiation, propagation, and termination.
- Learn exams of free radical reactions in industry and daily life: converting alkanes to haloalkanes, cracking process, combustion, polymerization, and the aging process.
Free radical reaction mechanism
Hemolytic bond breaking requires energy equal to the bond dissociation energy, and hemolytic bond making releases energy equal to the bond dissociation energy. When a molecule absorbs energy in the form of heat or a photon of UV light, some of the weak bonds, like \(\ce{Cl-Cl}\) bond (239 kJ/mol) or \(\ce{O-O}\) bond (146 kJ/mol) breaks initiating free radicals, e.g.:
 ,
,
where hv represents a photon of UV light and ∆ represent heat energy. The reaction in which free radicals are created from neutral species, as in the above example, is called the free radical initiation step in a free radical reaction mechanism.
Free radicals are very reactive species. They usually abstract an atom, e.g., an H atom, from a hydrocarbon molecule, e.g.:

This reaction happens easily because the energy needed to break an \(\ce{H-C}\) bond (413 kJ/mol) is compensated by the energy released by making \(\ce{H-Cl}\) bond (427 kJ/mol). The \(\ce{\stackrel{\bullet\;\;\;\;}{CH3}}\) repeats similar process when it collides with a \(\ce{Cl2}\) molecule as shown in the second reaction above. Again, the energy released by making \(\ce{C-Cl}\) bond (339 kJ/mol) compensates for the energy needed in breaking \(\ce{Cl-Cl}\) bond (239 kJ/mol). Reactions in which one radical converts into a neutral specie and create another free radical that repeats the process, as in the above two reactions, are called propagation reactions. The propagation reactions often happen easily as the energy released in the bond-making compensates fully or partially for the energy needed in bond-making. The propagation reactions shown above happen in a cycle as the free radical produced in one is the reactant in the other and vice versa. The two propagation steps add up to the following overall reaction:
Overall reaction: \(\ce{CH4 + Cl2 -> CH3Cl + HCl}\)
This cycle of propagation reaction may repeat hundreds of times until one of the reactant exhaust or a free radical may collide with another free radical and terminate each other, as in the following reactions.

The homolytic bond-making between two free radicals that terminate the two radicals is called the termination reaction. The initiation, propagation, and termination are typical elementary reactions in a free radical reaction mechanism.
A \(\ce{\stackrel{\bullet\;\;\;\;}{CH3}}\) may collide with \(\ce{CH4}\) molecule and abstract a \(\ce{H}\) atom and a \(\underset{\bullet\bullet}{_•^•\stackrel{\bullet}{Cl}{_•^•}}\) may collide with a \(\ce{Cl2}\) molecule and abstract a \(\ce{Cl}\) atom, but there is no net chemical change in these elementary steps as shown below.

Examples of free radical reactions
Conversion of alkanes to haloalkanes
Exposing a mixture of alkane and halogen (chlorine or bromine) to UV light or heat at ~100oC converts alkanes to haloalkanes, as described in the free radical reaction mechanism for the case of \(\ce{CH4}\) to \(\ce{CH3-Cl}\) conversion.
\[\ce{Cl2 + CH4 ->[\text{UV or heat}]CH3Cl + HCl}\nonumber \]
This reaction is useful in industrial organic synthesis for converting alkanes found in petroleum to alkyl halides that serve as intermediates in synthesizing organic compounds. The reaction is not very useful for laboratory organic synthesis as the product of the reaction competes with the initial alkane resulting in a mixture of products, as shown in the following reactions.
\[\ce{Cl2 + CH3Cl ->[\text{UV or heat}]CH2Cl2 + HCl}\nonumber \]
\[\ce{Cl2 + CH2Cl2 ->[\text{UV or heat}]CHCl3 + HCl}\nonumber \]
\[\ce{Cl2 + CHCl3 ->[\text{UV or heat}]CCl4 + HCl}\nonumber \]
The haloalkane product can not compete effectively when the initial alkane concentration is high. So, the side products are minimized in the industrial process by employing a higher concentration of alkane. This reaction converts relatively less reactive alkanes to more reactive haloalkanes having polar \(\ce{\overset{\delta{+}}{C}{-}\overset{\delta{-}}{Cl}}\) or \(\ce{\overset{\delta{+}}{C}{-}\overset{\delta{-}}{Br}}\) bond. The haloalkanes are used in the polar reactions described in later sections.
Combustion
Hydrocarbons found in petroleum, coal, and natural gas are primarily consumed in combustion processes for producing heat. In a combustion process, ground state \(\ce{O2}\) is first converted to an excited state \(\ce{O2^{\ast}}\), that strips a \(\ce{H}\) off of an organic molecule in the free radical initiation step.
\[\ce{R-CH3 + O2^{\ast} -> R-\!\!\stackrel{\bullet\;\;\;\;}{CH2}+ HO\!\!\stackrel{\bullet}{O}}\nonumber \]
The free radicals, i.e., \(\ce{R-\!\!\stackrel{\bullet\;\;\;\;}{CH2}}\) and \(\ce{HO\!\!\stackrel{\bullet}{O}}\) react further producing more radicals and, ultimately, convert the organic compounds to \(\ce{CO2}\), \(\ce{H2O}\), and heat. The combustion reaction of octane and ethanol are shown below as examples.
\[\ce{2CH3CH2CH2CH2CH2CH2CH2CH3 + 25O2 -> 16CO2 + 18H2O + {Heat}}\nonumber \]
\[\ce{CH3CH2OH + 3O2 -> 2CO2 + 3H2O + {Heat}}\nonumber \]
Cracking
 Although \(\ce{C-C}\) (347 kJ/mol) and \(\ce{C-H}\) (413 kJ/mol) bonds are stable at room temperature, at 450 oC to 900 oC there is enough thermal energy to break them homolytically. The resulting radical species react further and usually end up in smaller chain alkanes and alkenes, as illustrated in the figure on the right (Pengeldi, CC0, via Wikimedia Commons). This process is called thermal cracking. For example, one of the many reactions involved in the thermal cracking of octane is the following.
Although \(\ce{C-C}\) (347 kJ/mol) and \(\ce{C-H}\) (413 kJ/mol) bonds are stable at room temperature, at 450 oC to 900 oC there is enough thermal energy to break them homolytically. The resulting radical species react further and usually end up in smaller chain alkanes and alkenes, as illustrated in the figure on the right (Pengeldi, CC0, via Wikimedia Commons). This process is called thermal cracking. For example, one of the many reactions involved in the thermal cracking of octane is the following.

Catalysts and steam may be added to accelerate the sections and/or modify the nature of products. Cracking convert long-chain alkanes to more valuable small-chain alkanes and alkenes.
Polymerization
Polymers are long molecules composed of hundreds or thousands of repeat units called monomers. One polymer synthesis mechanism is free radical polymerization, involving initiation, propagation, and termination steps. The process initiates by homolytic cleavage of a weak bond, like \(\ce{O-O}\) bond in benzoyl peroxide (\(\ce{C6H5COO-OOCC6H5}\), by heat or UV-photon. The radical produced by the homolytic cleavage adds to a \(\pi\) bond of an alkene producing a new radical. The new radical adds to another alkene repeating the process in propagation steps, as illustrated below.

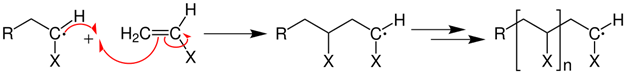 ,
,
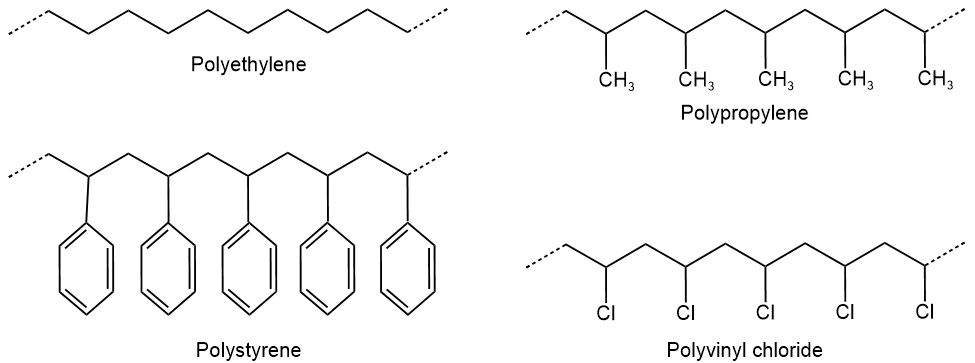
where \(\ce{X}\) is \(\ce{H}\) in polyethylene, \(\ce{CH3}\) in polypropylene, benzene ring \(\ce{C6H5}\) in polystyrene, \(\ce{Cl}\) in polyvinylchloride, etc., as illustrated below.
Ultimately, the radicals react with each other by hemolytic bond =-making that terminates the radicals.
Polyethylene is used in plastic bags, bottles, toys, piping, etc. Polypropylene is used in ropes, carpets, pipes, furniture, food containers, etc. Polystyrene is used in foam packing, building insulation foam, plastic cutlery, foam containers for food and drinks, etc. Polyvinylchloride is used in pipes, window and door frames, bank cards, cable insulation, etc. In polytetrafluorethylene or Teflon, all \(\ce{H's}\) and \(\ce{X}\) in the monomer are \(\ce{F's}\). Teflon is used in non-stick coatings, lubricants, electrical insulation for wires, etc. Various objects of common use made of these polymers are illustrated in Figure \(\PageIndex{1}\).

Free radical reactions in biological processes
Free radical reactions are involved in the aging of food products. For example, fatty acids have long alkane chains, many of which have \(\ce{C=C}\) bonds. The allylic hydrogens in fatty acid chains are susceptible to hemolytic bond cleavage, as shown below.

Oxygen is a diradical that adds to the free radical in a hemolytic bond-making step, producing a new radical. The new radical abstracts allylic hydrogen from another fatty acid producing a radical that repeats the cycle, i.e., propagation steps. The propagation reactions install hydroperoxyl groups (\(\ce{-O-OH}\)) on the fatty acids. The hydroperoxides are unstable and decompose to short-chain aldehydes and carboxylic acids responsible for the aged fats' unpleasant "rancid" smell. A similar process happens with the low-density lipoproteins deposited in arteries that lead to cardiovascular diseases. The aging process in organisms is also related to similar free radical reactions.
Living things have a mechanism of getting rid of unwanted free radicals by reacting them with radical scavengers. For example, vitamin C is a radical scavenger in the blood, and vitamin E is a radical scavenger in fats. The radical-scavengers neutralize the toxic radicals by donating \(\ce{H}\) atoms from their \(\ce{-OH}\) groups that break the propagation chain reaction. The new radicals generated in the scavenging reactions are less reactive and easily excreted before they can do more damage.
 Vitamin C
Vitamin C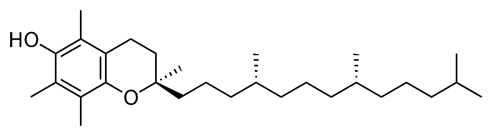 Vitamin E
Vitamin E

