8.4: Catalysis
- Page ID
- 357440
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
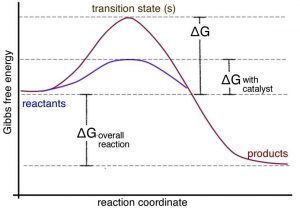
A catalyst provides an alternate pathway for a reaction to occur. More importantly, this pathway usually involves a lower activation energy than the uncatalyzed pathway, as shown in the graph. This means that the rate of the reaction can increase. It can do so because at a given temperature, collisions with enough energy to overcome the new lower activation energy barrier occur more frequently. But because the catalyst is neither a reactant nor a product of the reaction, it does not influence the reaction’s overall energy change. In biological systems, there are protein and RNA-based catalysts (enzymes and ribozymes); in non-living systems, minerals and metals often act as catalysts. Even simple species such as protons can be considered catalysts. Anything that is unchanged at the start and at the end of the reaction can be considered a catalyst. There are many different mechanisms through which catalysts can act. Biological catalysts are generally very selective in terms of the reactions they catalyze and very effective in speeding reactions up. It is not uncommon for the rate of a catalyzed reaction to be millions of times faster than the uncatalyzed reaction. In a complex reaction system, speeding up one reaction at the expense of others can have profound effects. However, there are also many examples where enzymes catalyze “off-target” reactions of the same or different types (although these reactions are generally accelerated to a much lesser extent). This ability to catalyze a range of reactions occurs because the surfaces of enzyme molecules are complex and often accommodate and bind a range of molecules. In other words, they are promiscuous.[13] The common analogy of an enzyme as a lock and the reactant molecules are viewed as the unique key, but this is far too simplistic. In reality, there are many molecules that can bind to a specific active site in an enzyme with greatly varying affinities. Although the mode of action of enzymes varies, in many cases the active site holds the two reactive molecules in close juxtaposition, which can speed their reaction. Can you imagine why?[14]
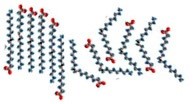
An organic chemical reaction that requires a catalyst is the addition of hydrogens across a \(\mathrm{C=C}\) bond. Without the catalyst, this reaction would not occur on a human timescale. It is an important reaction in many pharmaceutical syntheses and in the production of fat (solid) from oil (liquids). For example, margarine is produced by adding hydrogen to the \(\mathrm{C=C}\) bonds of oils extracted from plants, as shown in the figure. The removal of the \(\mathrm{C=C}\) bond makes the molecules pack better together. This is because London dispersion forces can now act upon the whole length of the molecule, increasing the strength of the van der Waals interactions between the molecules. Thus, the hydrogenated oil is a solid at room temperature. The catalyst is usually a transition metal, palladium (\(\mathrm{Pd}\)) or platinum (\(\mathrm{Pt}\)), finely divided and adsorbed onto the surface of an inert substance like charcoal (carbon), as shown in the figure. The transition metal has empty \(\mathrm{d}\) orbitals that interact with the \(\mathrm{C=C}\) bond’s pi orbital, destabilizing the pi bond and making it more susceptible to reaction. \(\mathrm{H}_{2}\) molecules also adsorb onto (interact with) the surface of the transition metal and insert themselves between the \(C\) and the catalyst, forming a fully-hydrogenated fat. Unfortunately, in many cases the hydrogen does not add across the double bond. Instead, the bond isomerizes from cis to trans, forming the unnatural trans isomer which has been implicated in the development of heart disease.[15]
Questions to Answer
- Draw a representation of an enzyme active site. What kinds of interactions do you think hold the substrate molecule in the active site?
- Why do you think binding two reactants in close proximity will increase the reaction rate?


