Extra Credit 24
- Page ID
- 82994
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.3.3
Determine the overall reaction and its standard cell potential at \(25 ^\circ C\) for this reaction. Is the reaction spontaneous at standard conditions?
\[Cu(s)\vert Cu^{2+}(aq) \vert\vert Au^3+(aq) \vert Au(s)\]
S17.3.3
The left side of the double bar is the anode and the right side is the cathode. The anode is where oxidation occurs (loss of electrons) and the cathode is where reduction occurs (gain of electrons)
First split the equation into two different redox half reactions: one oxidation and one reduction equation.
Oxidation: \[Cu(s) \rightarrow Cu^{2+}(aq)\]
Reduction: \[Au^{3+}(aq) \rightarrow Au(s)\]
Balance each half reaction
\[Cu(s) \rightarrow Cu^{2+}(aq) + 2e^-\]
\[3e^-+Au^{3+}(aq) \rightarrow Au(s)\]
Multiply each equation so that they have the same number of electrons
\[3Cu(s) \rightarrow 3Cu^{2+}(aq)+6e^-\]
\[6e^-+2Au^{3+}(aq) \rightarrow 2Au(s)\]
Cancel electrons and add equations
\[3Cu(s) \rightarrow 3Cu^{2+}(aq)+6e^-\]
\[6e^-+2Au^{3+}(aq) \rightarrow 2Au(s)\]
\[3Cu(s)+2Au^{3+}(aq) \rightarrow 3Cu^{2+}(aq)+2Au(s)\]
To find its standard potential you will need a standard cell potential chart for \(25^\circ C\).
The equation for calculating the standard potential of a cell is:
Cell potential = (cell potential cathode - cell potential anode)
*Do not pay attention to the direction of the reaction, just the cell potential
According to the table:
cell potential cathode = \(1.5 V\)
cell potential anode = \(0.34 V\)
standard cell potential = \((1.5V - 0.34V)\) = \(1.16 V\)
If the standard cell potential is positive, then it is a spontaneous reaction. If it is negative, it is non spontaneous.
Reaction is spontaneous
Answer:
\(3Cu(s)+2Au^{3+}(aq)\)→ \(3Cu^{2+}(aq)+2Au(s)\) ; \(+1.16V\); spontaneous
Q19.1.22
Balance the following equations by oxidation-reduction methods; note that three elements change oxidation state.
\[Co(NO_3)_2(s) \rightarrow Co_2O_3(s)+NO_2(g)+O_2(g)\]
S19.1.22
We can actually solve this problem using normal methods of balancing equations.
But you can balance it normally.
1.) First, write the unbalanced equation with reactants on the left, products on the right and and arrow in between.
\[Co(NO_3)_2(s) \rightarrow Co_2O_3(s)+NO_2(g)+O_2(g)\]
2.) Start by balancing elements that appear only once on each side of the reaction by placing a coefficient in front of the species. For example, balance cobalt first.
\[2Co(NO_3)_2(s) \rightarrow Co_2O_3(s)+NO_2(g)+O_2(g)\]
3.) Balance the other element that only appears once. Balance nitrogen
\[2Co(NO_3)_2(s) \rightarrow Co_2O_3(s)+4NO_2(g)+O_2(g)\]
4.) Finally, balance the last element, oxygen. Note: you may have to change the coefficients of other species in order to balance the oxygens.
\[4Co(NO_3)_2(s) \rightarrow 2Co_2O_3(s)+8NO_2(g)+O_2(g)\]
Answer:
\[4Co(NO_3)_2(s) \rightarrow 2CO_2O_3(s)+8NO_2(g)+O_2(g)\]
Q12.4.14
There are two molecules with the formula \(C_3H_6\). Propene, \(CH_3CH=CH_2CH_3CH=CH_2\), is the monomer of the polymer polypropylene, which is used for indoor-outdoor carpets. Cyclopropane is used as an anesthetic:
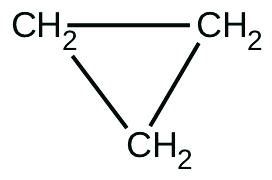
When heated to \(499 ^\circ C\), cyclopropane rearranges (isomerizes) and forms propane with a rate constant of \(5.95 \times 10^{-4} s^{-1}\). What is the half-life of this reaction? What fraction of the cyclopropane remains after \(0.75\) h at \(499 ^\circ C\)?
S12.4.14
What is the half life of this reaction?
This is a first order reaction.
1.) Equation:
\(t1/2 = ln2/k\)
\(t1/2 = half life; k =\) rate constant
2.) Plug in values
\(t1/2 = ln2/(5.95\times10^{-4} s^{-1})\)
\(t1/2 = 1165 s\) or \(0.32 h\)
What fraction of cyclopropane remains after \(0.75 h\) at \(499.5 ^\circ C\)?
The temperature of the reaction does not affect the half-life of the material. So, we do not need to use the temperature in the calculations.
1.) Equation (first order):
\[ln([A]t/[A]_0) = −kt\]
2.) Initial and final concentration are unknown. Substitute \(x\) for \(([A]t/[A]_0)\)
\[ln(x)=-kt\]
3.) Since k is time time convert \(0.75 h\) into seconds.
\[t= 0.75 h \times (60 min/h) \times (60 s/min) = 2,700 s\]
4.) Plug values into equation
\[ln(x) = -5.95\times10^{-4}s^{-1}(2 700 s)\]
5.) Simplify and solve for \(x\)
\[e^{lnx} = e^{-1.6065}\]
\[ x = 0.20\]
6.) Multiply by 100% to find how much is left
\[0.20 \times 100\text% = 20 \text%\]
\(1165 s; 20 \text%\)
Q21.2.9
Which of the following nuclei lie within the band of stability?
- chlorine-\(37\)
- calcium-\(40\)
- \(^{204}\)Bi
- \(^{56}\)Fe
- \(^{206}\)Pb
- \(^{211}\)Pb
- \(^{222}\)Rn
- carbon-\(14\)
S21.2.9
The band or belt of stability helps to determine which isotopes are stable and which are unstable. Unstable means that the species is likely to go under some form of decay. The \(x\)-axis of the graph is the proton number (Z) and \(y\)-axis is the neutron number (N)

a. chlorine-\(37\)
1.) Find number of protons. Atomic number = # protons
Cl has \(17\) protons
2.) Find number of neutrons.
(number of neutrons = mass number - atomic number)
number of neutrons = \(37 - 17\)
number of neutrons = \(20\)
3.) Use number of protons (\(x\)-value) and number of neutrons (y-value) Point: (17,20)
4.) Determine if point is on the band of stability.
\((17,20)\) is within the band of stability so chlorine-\(37\) is within the band of stability
b. calcium-\(40\)
1.) \(20\) protons
2.) \(20\) neutrons
3.) Point: \((20,20)\)
4.) calcium-\(40\) is within the band of stablility
c. \(^{204}\)Bi
1.) \(83\) protons
2.) \(121\) neutrons
3.) \((83,121)\)
4.) \(^{204}\)Bi is within the band of stability
d. \(^{56}\)Fe
1.) \(26\) protons
2.) \(30\) neutrons
3.) \((26,30)\)
4.) \(^{56}\)Fe is within the band of stability
e. \(^{206}\)Pb
1.) \(82\) protons
2.) \(124\) neutrons
3.) \((82,124)\)
4.) \(^{206}\)Pb is within the band of stability
f. \(^{211}\)Pb
1.) \(82\) protons
2.) \(129\) neutrons
3.) \((82,129)\)
4.) \(^{211}\)Pb is above the band of stability
g. \(^{222}\)Rn
1.) \(86\) protons
2.) \(136\) neutrons
3.) \((86,136)\)
4.) \(^{222}\)Rn is above the band of stability
h. carbon-\(14\)
1.) \(6\) protons
2.) \(8\) neutrons
3.) \((6,8)\)
4.) carbon-\(14\) is below the band of stability
answer: a,b,c,d,e
Q21.7.1
If a hospital were storing radioisotopes, what is the minimum containment needed to protect against:
1.) cobalt-\(60\) (a strong \(\gamma\) emitter used for irradiation)
2.) molybdenum-\(99\) (a beta \(\beta\) emitter used to produce technetium-\(99\) for imaging)
S21.7.1
1.) cobalt-\(60\) (a strong \(\gamma\) emitter used for irradiation)
Gamma rays or \(\gamma\) are the strongest form of radiation. They have no mass and no charge. Gamma rays can pass through paper, human skin, sheets of metal, water, and concrete but are stopped by lead. For this reason, the hospital would need to store the cobalt-\(60\) in a container made out of lead.
2.) molybdenum-\(99\) (a beta \(\beta\) emitter used to produce technetium-\(99\) for imaging)
Beta emissions are stronger forms of radiation than alpha particles, but are weaker than gamma rays. Beta particles can go through paper and human skin, but can be stopped by sheets of metal. For this reason, molybdenum-\(99\) should be stored in the hospital in a container made out of aluminum or some similar material.

Q20.4.10
For each application, describe the reference electrode you would use and explain why. In each case, how would the measured potential compare with the corresponding E°?
a.) measuring the potential of a \(Cl^-/Cl_2\) couple
b.) measuring the \(pH\) of a solution
c.) measuring the potential of a \(MnO_4^-/Mn^2+\) couple
S20.4.10
For each application, describe the reference electrode you would use and explain why. In each case, how would the measured potential compare with the corresponding E°?
A reference electrode is one that has a constant and stable potential. You need different reference electrodes depending on what you are measuring.
a.) measuring the potential of a \(Cl^-/Cl_2\) couple
1.) obtain a standard reduction potential table
2.) Find reaction on table and corresponding \(E^\circ\):
\[Cl_2(g) + 2e^- \rightarrow 2Cl^-; 1.36 V\]
3.) Flip equation to match reaction in question
\[2Cl^- \rightarrow Cl_2(g) + 2e^-; -1.36 V\]
(flipping reaction will result in a change in sign of \(E^\circ\)Determine whether reaction is occurring at the anode or cathode Reaction has electrons in products so it is losing electrons therefore it is occurring at the anode.
Since the electrode accepts electrons from solution at the anode, we need an electrode that will accept electrons. The more positive \(E^\circ\) is the more likely is to accept electrons. Zinc has a higher standard potential than \(-1.36\) so it can act as the electrode for this reaction. (multiple answers)
The potential of the zinc reference electrode is \(-0.76 V\) so the difference between that and \(Cl^-/Cl_2\) is \(0.6V\)
b.) measuring the \(pH\) of a solution
To measure \(pH\) of a solution, you would need an ion-selective electrode. Ph electrodes are sensitive to \(H^+\) ions.
On the reduction potential table
\[H_2 → 2H^+, E^\circ =0\]
The material in the \(pH\) electrode can either have an \(E^\circ\) either larger than \(0\) or less than \(0\). So we can pick basically any reasonable metal. A popular metal to put in \(pH\) electrodes is silver.
The potential of a silver reference electrode is \(0.8V\) so the difference between that and \[H_2 → 2H^+\) is \(0.8V\)
c.) measuring the potential of a \(MnO_4^-/Mn^2+\) couple
Reaction from table (no need to flip):
\[MnO_4^{-} + 8H^{+} + 5e^{-} \rightarrow Mn^{2+} + 4H_2O\]
\[E^\circ = +1.5\]
This is a reduction reaction so it will occur at the cathode. The electrode at the cathode donates electrons. The more negative the \(E^\circ\), the more likely that an electron will be donated. Copper solid would be a good electrode in this case because it has a more negative \(E^\circ\).
The potential of a copper reference electrode is \(0.34V\) so the difference between that and \(MnO_4^-/Mn^2+\) id \(1.16V\)
Q20.4.11
Draw the cell diagram for a galvanic cell with an SHE and a copper electrode that carries out this overall reaction:
\[H_2(g)+Cu^{2+}(aq) \rightarrow 2H^+(aq)+Cu(s)\]
S20.4.11
Draw the cell diagram for a galvanic cell with an SHE and a copper electrode that carries out this overall reaction:
\[H_2(g)+Cu^{2+}(aq) \rightarrow 2H^+(aq)+Cu(s)\]
A galvanic cell is one that describes energy from spontaneous redox reactions happening within the cell.

SHE stands for standard hydrogen electrode. The standard is determined by the potential of a platinum electrode. So, the electrodes for this system will be \(Cu(s)\) and \(Pt(s)\).
First, we have to figure out which element is being oxidized and which is being reduced using the oxidation states of the elements. \(H\) is going from \(0\) to \(+1\) and \(Cu\) is going from \(+2\) to \(0\). So, hydrogen is being oxidized and copper is being reduced.
"Anode" is where oxidation occurs and is typically written on the left side of the cell.
"Cathode" is where reduction occurs and is typically written on the right side of the cell.
Changes in state are denoted by a single vertical bar and the salt bridge is denoted by two vertical bars. The electrodes are always written on either side of the cell diagram. Make sure to include states.

The anode includes the \(H_2(g)\), \(H^+(aq)\) and \(Pt(s)\) electrode.
The cathode includes the \(Cu^{2+}(aq)\) and \(Cu(s)\) electrode.
Cell diagram:
\(Pt(s)\vert H_2 (g, 1 atm),\vert H^+(aq, 1M) \vert\vert Cu^{2+}(aq, 1M) \vert Cu(s)\)
Q20.8.2
What does it mean when a metal is described as being coated with a sacrificial layer? Is this different from galvanic protection?
S20.8.2
What does it mean when a metal is described as being coated with a sacrificial layer? Is this different from galvanic protection?
Corrosion is the unwanted oxidation of a material. This is a natural process which converts a refined metal to a more chemically stable form. Both sacrificial layers and galvanic protection prevent corrosion. One can prevent corrosion by adding a protective layer. A layer of zinc can be added to steel to prevent corrosion. This is considered an example of galvanic protection. A sacrificial layer is essentially the same thing. When a metal is coated with a sacrificial layer, it simply means that the sacrificial layer is more likely to be oxidized, and thus will protect whatever it is covering.

