Extra Credit 15
- Page ID
- 83522
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q19.4C
Sketch a voltaic cell for each of the following conditions, labeling the anode, cathode, and electron flow. Balance the redox equation if necessary. Calculate \(\mathrm{E^\circ_{cell}}\) for each cell.
- \(\mathrm{Cu^{2+}(aq) + Sn^{2+}(aq) \rightarrow Cu(s) + Sn^{4+}(aq)}\)
- \(\ce{Mg(s)}\) displaces \(\ce{Sn^2+(aq)}\) from solution
- \(\ce{Zn(s)}\) donates e- to acidic solution to form \(\ce{H2(g)}\)
- \(\mathrm{Pb^{2+}(aq) +Sn(s) \rightarrow Pb(s) + Sn^{2+}(aq)}\)
S19.4C
For this question we will need to look at a table of Potential standards in order to calculate \(\mathrm{E^\circ_{cell}}\).
Remember when balancing redox reactions to balance the trading of electrons.
To calculate \(\mathrm{E^\circ_{cell}}\) we need to use the equation \(\mathrm{E^\circ_{cell}}\)= \(\mathrm{E^\circ}\) of Reduction (cathode) - \(\mathrm{E^\circ}\) of Oxidation (anode).
So we look at the table and come to the conclusion that the \(\mathrm{E^\circ_{cell}}\)'s are equal to:
- \(\mathrm{E^\circ_{cell}}\) of \(\mathrm{Cu^{2+}(aq) + Sn^{2+}(aq) \rightarrow Cu(s) + Sn^{4+}(aq)}\)=.4959
- \(\mathrm{E^\circ_{cell}}\) of \(\mathrm{Mg(s)+Sn^{2+}(aq){\rightarrow}{Sn(s)+Mg^{2+}(aq)}}\)=2.216
- \(\mathrm{E^\circ_{cell}}\) of \(\mathrm{Zn(s)+2H^{+}(aq){\rightarrow}{Zn^{2+}(aq)+H_2(g)}}\)=-.7618
- \(\mathrm{E^\circ_{cell}}\) of \(\mathrm{Pb^{2+}(aq) +Sn(s) \rightarrow Pb(s) + Sn^{2+}(aq)}\)=-.266

Q19.41C
If \({\mathrm{[Cu^{2+}]}}\) is maintained at 2.0 M
What is the minimum \({\mathrm{[Ag^+]}}\) must be at to push the forward direction spontaneously. Use the equation
\({\mathrm{Cu(s)}+2{Ag^+(aq)}{\rightarrow}{Cu^{2+}}(aq)+{2Ag(s)}}\)
S19.41C
For this question we will need to look at a table of Potential standards in order to calculate \(\mathrm{E^\circ_{cell}}\).
To push a reaction forward spontaneously, the \({\mathrm{E_{cell}}}\) must be greater than 0. We find the \({\mathrm{E^\circ_{cell}}}\)= 0.460 V.
Next, plug in the values given to the Nernst equation. The Nernst equation is as follows:
E=\(\mathrm{E^{\circ}}-\dfrac{RT}{nF}ln\dfrac{[Reduction]}{[Oxidation]}\)
The number of moles of electrons being transferred is 2. The equation will be set up as: \({\mathrm{0 = 0.460- \dfrac{.0592}{2}\times\log\dfrac{2}{x^2}}} \)
Remember to account for coefficients in setting up the \(\mathrm{Q}\). Now solve for x \(\ce{[Ag+]}\) and we find that \(\mathrm{x = 2.4 \times 10^{-8}\, M}\).
For review on this topic, visit the page "Electrochemistry 4: The Nernst Equation".
Q20.57A
Predict the molecular formulas for the following metal carbonyls and explain how many electrons are contributed by the metal and how many are contributed by the carbon monoxide and which noble gas electron configuration does the molecule exhibit.
- Titanium (T)
- Manganese (Mn)
- Tungsten (W)
S20.57A
Each element needs to complete its valence octet so it needs the number of CO molecules to help it complete it. Noble gases have complete octets so when an element has a complete octet it will have the same electron configuration as a noble gas.
- \({\mathrm{Ti(CO)_7}}\); Ti has 22 electrons and 7 CO contribute 14 electrons for a total of 36 electrons which the same number of electron as Kr.
- \({\mathrm{Pt(CO)_4}}\); Pt has 78 electrons and 4 CO contribute 8 electrons for a total of 86 electrons which is the element Rn.
- \({\mathrm{W(CO)_6}}\); W has 74 electrons and 6 CO contribute 12 electrons for a total of 86 electrons which is the element Rn.
Q24.4A
In the reaction \(\mathrm{A \rightarrow products}\), at \(\mathrm{t = 0}\), \(\mathrm{[A]=0.1563\,M}\). After 1.00 minute, \(\mathrm{[A]=0.1496\,M}\), and after 2.00 minutes, \(\mathrm{[A]=0.1431\,M}\).
- Calculate the average rate of reaction during the first minute and during the second minute.
- Why are these two rates not equal?
S24.4 A
Top find the rate of reaction we need to use the following equation:
rate= \(\mathrm{\dfrac{\Delta concentration}{\Delta time}}\)
- First minute: rate of reaction= \(\mathrm{-0.0067\:M/min}\) Second minute: rate of reaction= \(\mathrm{-.0065\:M/min}\)
- The rates are not equal because the rate of the reaction depends on the order of the reaction. The order of the reaction can't be zero because the change in concentration has an effect on the rate of the reaction. If the reaction rate was zero there would be now effect on the rate of reaction when the concentration changes.
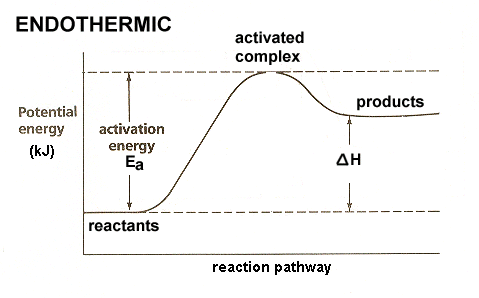
Q24.47B
For a reversible reaction, the enthalpy change of the forward reaction is 37kJ/mol, and the activation energy of the forward reaction is 96kJ/mol.
- With the information provided, are you able to determine the activation energy of the reverse reaction? If so, please determine it.
- Provide a sketch of the potential energy vs. progress of reaction.
S24.47B
- Yes we are able to determine the activation energy of the reverse reaction. Because the enthalpy change of the forward reaction is 37kJ/mol, the products are 37kJ/mol closer to the transition state than the reactants. The activation energy for the reverse reaction is \(\mathrm{96\,kJ/mol-37\,kJ/mol=59\,kJ/mol}\).
- \(\mathrm{\Delta H=+37\,kJ}\)
\(\mathrm{E_a(forward)=96\,kJ}\)
\(\mathrm{E_a(reverse)=59\,kJ}\)
Q25.25B
If a sample containing \(\ce{^{33}_{16}S}\) is having activity 100 times the detectable limit. How long will the experiment need to be run for this sample before the radioactivity could not detected? Assume half life of \(\ce{^{33}_{16}S}\) is 14.3 day.
S25.25B
\(\ce{^{33}_{16}S}\) half life is given. Since we need to determine the time require getting to the detectable limit. \(\dfrac{1}{100}\) of the initial value. Hence we use
\[\mathrm{K= \dfrac{ln(2)}{t_{1/2}}}\], where \(\mathrm{K}\) is the decay constant, and \(\mathrm{t_{1/2}}\) is equal to half life. We then plug those in and get:
\[\mathrm{K = \dfrac{0.693}{t_{1/2}}=\dfrac{0.693}{14.3\, d}=0.0485\,d^{-1}}\]
\[\mathrm{\ln \left (\dfrac{1}{100}\right) =-0.0485\,d^{-1}\left ( t \right )}\]
\[\mathrm{T=\dfrac{\ln\left(\dfrac{1}{100}\right )} {-0.0485\,d^{-1}}=94.95=95\,days}\]
Q18.2
In the oxidation-reduction reaction,
\[\ce{Br2(l) + 2 I- (aq) \rightarrow 2 Br- (aq) + I2(s)}\]
- which substance(s) is being reduced?
- which element(s) is increasing in oxidation number?
- which element(s) is gaining electrons?
- which substance(s) is the oxidizing agent?
- which substance(s) is the reducing agent?
S18.2
\[\ce{Br2(l) + 2 I- (aq) \rightarrow 2 Br- (aq) + I2(s)}\]
For this reaction, and to answer the questions we need to look at oxidation numbers. Oxidation numbers are relative to the corresponding ion's charge. Therefore, \(\mathrm{Br_2}(l)\) has an oxidation charge of 0, \(\mathrm{I^-}\)(aq) has an oxidation number of -1, \(\mathrm{Br^-}\)(aq) has an oxidation number of -1, and \(\mathrm{I_2(s)}\) has an oxidation number of 0. For whether or not an element is oxidizing or reducing we can think of O.I.L.R.I.G. which stands for Oxidation is loss Reduction is gain. So oxidation is a loss of electrons while reduction is gain of electrons. To determine the reducing agent and oxidizing agent you can think of the opposite. So if something is being reduced it is the oxidizing agent and visa versa. There fore the answers are as follows:
1. \(\mathrm{Br_2}\)
2. I
3. Br
4. \(\mathrm{Br_2}\)
5. \(\mathrm{I^-}\)
Q21.3.8
At the end of a star’s life cycle, it can collapse, resulting in a supernova explosion that leads to the formation of heavy elements by multiple neutron-capture events. Write a balanced nuclear reaction for the formation of each isotope during such an explosion.
- \({\mathrm{^{106}Pd}}\) from nickel-58
- selenium-79 from iron-56
S21.3.8
Since these elements are gaining neutrons and changing to a different atoms we need to balance out the equations using neutrons and beta particles which look like as follows;
\({^1_0n}\) \({^0_{-1}\beta}\)
So we use the beta particle to balance out the protons of the atom and we use the neutrons to balance out the weight of the atom and we get the answers:
1. \(\mathrm{^{58}_{28}Ni+48{^1_0n}}{\rightarrow}{^{106}_{46}Pd+18{^0_{-1}\beta}}\)
2. \(\mathrm{^{56}_{26}Fe+23{^1_0n}}{\rightarrow}{^{79}_{34}Se+8{^0_{-1}\beta}}\)

