Extra Credit 34 - Sarah Weber
- Page ID
- 82844
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.5.2
List some things that are typically considered when selecting a battery for a new application.
A battery is a self-contained unit that produces electricity, "contained" meaning it generates its own electrical energy internally. Thus, batteries serve the purpose of keeping many appliances/devices without an external power source running. Since batteries are contained, we have been able to make the devices that run on them portable, and being able to use such devices while on the go has made the lifestyle of this day and age possible.
Overall, considerations in the selection of batteries include:
Cost (Self-explanatory, being cost-efficient is a general goal)
Toxicity - How much will a given battery harm the environment?
Energy - How much energy does a battery emit, and is this energy efficient in achieving the task that the battery is "setting out" to accomplish?
Mass - How large is the battery, and will its mass affect its ability to work?
Lifetime - How long does it last?
Different types of batteries embody these traits in different ways, and thus, the setting/purpose in which one uses a battery greatly determines what type of battery will be best used in a given application. In other words, the type of battery is definitely a consideration when selecting a battery for a given application.
This being said, let's look at types of batteries and see how each type's characteristics play a role in where it can be applied...
Primary Batteries
- Non-rechargeable
- Irreversible reactions take place and hence cannot be reused
- Cheap
- Common in everyday appliances
Secondary Batteries
- Rechargeable (click on hyperlink for more on rechargeable batteries)
- If one were to apply an electrical potential (in the reverse direction of the initial reaction) to a secondary battery, they can reuse the battery! (this is what happens when one "charges" a secondary battery; they quite literally apply a "charge" to the cell)
- More expensive
- Often used in automobiles
Fuel Cells
- *Technically not a "battery" since they are not self-contained; they use an external source such as Hydrogen in order to produce energy
- Most efficient in producing electricity
- Most expensive
- Used in U.S. space vehicles (yes, they generate that much energy! ... and it is rocket science)
*Refer to Section 19.5 to get a full overview of types of batteries
Q12.2.1
Describe the effect of each of the following on the rate of the reaction of magnesium metal with a solution of hydrochloric acid: the molarity of the hydrochloric acid, the temperature of the solution, and the size of the pieces of magnesium.
Molarity - Molarity is defined as being the number of moles of solute divided by the number of liters of a given solution. This being said, if there is to be more molecules in a confined area (in other words, if a compound has a high molarity,) will these molecules collide with each other more or less than if there were less molecules? (the molecules will collide more!) Having more collisions yields a higher reaction rate.
Temperature - Temperature is what determines how fast a given compound's atoms/molecules move. Absolute zero, the absolute "coldest" that something can be, is defined by 0 particle movement in a compound. When one heats something up, however, that given compound's particles are going to move faster with increasing temperature. With these particles moving faster, there will be a higher chance of them colliding with one another, and thus, the reaction rate will increase.
Size of Pieces - The size of the pieces of magnesium determine how much surface is available to react, that is, how much of it is "exposed" to be reacted with. Smaller pieces will react more quickly since reactants can "get to" spots on a smaller piece of magnesium faster than a larger piece.
*Supplementary Source: See Section 14.1 regarding factors that affect reaction rates for confirmation of the above conclusions.
Q12.5.6
How does an increase in temperature affect rate of reaction? Explain this effect in terms of the collision theory of the reaction rate.
When observing how temperature affects the rate of a reaction, looking at the concepts behind collision theory helps one to draw a conclusion. Collision theory states that having more molecular collisions yields a quicker reaction since molecules are spending more time in contact with one another.
When relating this concept to temperature, raising the temperature of something makes the particles of any given compound move more quickly, meaning that they will collide more with one another. This being said, increasing temperature speeds up the rate of a reaction since there will be more collisions, and again, as the collision theory states, having more collisions leads to a quicker reaction rate.
Q21.4.1
What are the types of radiation emitted by the nuclei of radioactive elements?
Nuclear radioactivity is a topic that steers away from "regular chemistry" which concerns the activity of electrons, and instead, falls under the realm of "nuclear chemistry" which concerns the activity of nucleus.
When we look at the chemistry of the nucleus, we are mainly concerned with two particles - protons and neutrons (both of which make up the nucleus). Unlike electrons that have negligible mass in the context of chemical reactions, protons and neutrons possess a mass value that must be accounted for, and thus, in nuclear chemistry, chemical equations often include the atomic and mass numbers of a given element.
Another important facet of nuclear chemistry is the concept of spontaneity which tells one if a nuclear reaction takes place naturally (without any external intervention, a spontaneous reaction) or artificially (with an external trigger, a nonspontaneous reaction).
Listed below are the types of nuclear radiation, and since nuclear chemistry concerns protons and neutrons, each type of radiation can be measured by the loss/gain of each of these particles. (NOTE: Like any reaction, mass throughout the course of the reaction cannot be created or destroyed, and thus, the added atomic and mass numbers on each side of the equation should be equal to the other side.)
Alpha (α) Decay
- Atomic # - Decreases by 2
- Mass # - Decreases by 4 (2 of these units are from the 2 protons emitted, and thus, the other 2 units are neutrons)
- Charge - +2
- Symbol - \(^{4}_{2}\textrm{He}\)
- Oftentimes alpha decay occurs to nuclei with mass numbers greater than 200
- Results in the emission of a Helium-4 nucleus as an alpha particle
See below a balanced equation of alpha decay: \[^{233}_{90}\textrm{Th}\rightarrow^{229}_{88}\textrm{Ra} +^{4}_{2}\alpha\]
Beta (β-) Decay (electron)
- Atomic # - Increases by 1
- Mass # - Stays the same (an unstable neutron becomes a proton, so the overall mass stays constant throughout this whole ordeal!)
- Charge - -1
- Symbol - \(^{0}_{-1}\beta\)
- Often among atoms that contain "too many" neutrons (See Section 5.5 for concepts on atomic stability, the atom is trying to achieve "the stable" number of neutrons by ridding itself of one)
See below for a balanced equation of beta decay with an electron emitted: \[^{14}_{6}\textrm{C}\rightarrow^{14}_{7}\textrm{N} + ^{0}_{-1}\beta\]
Beta (β+) Decay (positron)
- Atomic # - Decreases by 1
- Mass # - Stays the same (an unstable proton becomes a neutron)
- Charge - +1
- Symbol - \(^{0}_{+1}\beta\)
- Often among atoms that contain "too little" neutrons (See Section 5.5 for concepts on atomic stability, the atom is trying to achieve "the stable" number of neutrons by gaining one)
See below for a balanced equation of beta decay with a proton emitted: \[^{14}_{6}\textrm{C}\rightarrow^{14}_{5}\textrm{B} + ^{0}_{+1}\beta^+\]
Electron Capture (EC)
- Atomic # - Decreases by 1
- Mass # - Stays the same (an electron from the atom's inner shell reacts with a proton to become a neutron)
- Charge - -1
- Symbol - \(^{0}_{-1}\textrm{e}\)
- Like β+ decay, EC often occurs among atoms that contain too little neutrons
- *It is important to note that the electrons in EC originate from the atom in which EC is occurring and that EC is not achieved from an act of deliberately firing electrons at a nucleus (artificial radiation)
See below for a balanced equation of EC: \[^{55}_{26}\textrm{Fe} + ^{0}_{-1}\textrm{e}\rightarrow^{55}_{25}\textrm{Mn} + \textrm{x-ray}\]
Gamma (γ) Decay
- Atomic # - Stays the same
- Mass # - Stays the same
- Charge - 0
- Symbol - \(^{0}_{0}\gamma\)
- Gamma decay is what occurs when a nucleus is in its excited state and releases energy in the form of a photon in order to achieve the stability of a ground state nucleus
- Gamma decay often occurs simultaneously with alpha decay since nuclei undergoing decay are typically excited
- In the above scenarios, gamma decay is spontaneous
- The symbol "*" indicates an excited state nucleus
See below for a balanced equation of gamma decay: \[^{234}_{90}\textrm{Th*}\rightarrow^{234}_{90}\textrm{Th} + ^{0}_{0}\gamma\]
Q20.2.5
Of the following elements, which would you expect to have the greatest tendency to be oxidized: Zn, Li, or S? Explain your reasoning.
Elements with the greatest tendency to oxidized are those that are most likely to have their electrons "stripped away." The likelihood of an element being oxidized increases as one goes left and down across the periodic table; left because elements there "wish to" give up their electrons to achieve a perfect eight valence shell, and down due to the shielding effect of larger elements. Shielding causes these elements to have less "pull" on their outermost electrons, and thus, they are easier to take away.
The phenomena described above are depicted below in the Pauling Electronegativity Chart

Pauling Electronegativity Values of the s-, p-, d-, and f-Block Elements. Values for most of the actinides are approximate. Elements for which no data are available are shown in grey. Source: Data from L. Pauling, The Nature of the Chemical Bond, 3rd ed. (1960).
This being said, if one looks at a periodic table, one can observe that Li lies in Group 1, which is the furthest to the left of the three elements, and is only one electron away from achieving a full outermost shell (it forms 1+ ions.) Li will "most happily" give away its electron, and as a result has the greatest tendency to be oxidized. *Check out the trends of alkali metals for confirmation that Li has a high ionisation energy, and thus, oxidation tendency.
Zn has the second greatest oxidation tendency of these three elements since it lies in the transition metal group, which is right of Li, and forms 2+ ions. Zn needs to give up two electrons to achieve a perfect outermost shell while Li only needs to give up one, so, it is slightly more difficult to oxidize Zn. *See the oxidations of transition metals to confirm this information.
S has the least tendency to be oxidized since it is more likely to be reduced; S will want to "snatch" electrons from other elements in order to achieve a full valence shell. S forms 2- ions because it needs two electrons to fill its outermost shell, and it would not want to give up electrons since it would fall farther away from the goal of a full shell. *See the article halogens as oxidizing agents for confirmation of the high electronegativity of S.
Q20.4.24
Your lab partner wants to recover solid silver from silver chloride by using a 1.0 M solution of HCl and 1 atm H2 under standard conditions. Will this plan work?
Before we go about solving this, let's write out the balanced chemical equation for the above reaction to have a better scope on what's going on: \[{2}\textrm{AgCl} +\textrm{H}_2\rightarrow{2}\textrm{HCl} + {2}\textrm{Ag}\]
In order for this equation to happen, H2 needs to be able to "kick out" Ag from AgCl, which will thus allow for the formation of solid silver in the products of this reaction How do we know if this can happen though? ... Standard reduction potentials!
The standard reduction potential of a reaction is determined by how likely a chemical species is to be reduced. We can compare the standard reduction potentials of the half-reaction equations to determine which element in our original equation will be the reducing agent and does the "kicking out."
\[\textrm{AgCl} +\textrm{e}^-\rightarrow\textrm{Ag} + \textrm{Cl}^-\]
\[{2}\textrm{H}^+ + {2}\textrm{e}^-\rightarrow\textrm{H}_2\]

If we use our derived half-reaction formulas and look for them in the standard reduction potential table, we can see that the half-reaction equation for AgCl has a more positive "reduction potential," meaning that it is more likely to be reduced in a reaction with H2 . Therefore, we can conclude that H2 will in fact "kick out" Cl- from AgCl and act as the reducing agent in this chemical reaction. The result is H2 becoming oxidized as it is now bound to Cl- (0 charge to +1 charge) and Ag becoming reduced as it now stands alone as solid silver (+1 charge to 0 charge).
So yes, the lab partner's method of recovering silver will work!
Q20.9.9
What mass of PbO2 is reduced when a current of 5.0 A is withdrawn over a period of 2.0 h from a lead storage battery?
In order to determine the mass of PbO2, one must first be able to recognize that this problem is on the topic of electroplating. However, one can't really recognize that this is an "electroplating problem" if they don't know what electroplating is first huh?
Electroplating is the process in which a layer of metal is deposited on the metal electrode that acts as the cathode during electrolysis. Electroplating serves the purpose of not only making objects look pretty but also protecting these objects from corrosion.
Now this is all fine and dandy, but how do we figure out how much metal is deposited through the process of electroplating? ... Stoichiometry!
(Quoted from Section 19.7) If we know the stoichiometry of an electrolysis reaction, the amount of current passed, and the length of time, we can calculate the amount of material consumed or produced in a reaction.
This being said, there are two formulas relating the stoichiometry of an electroplating scenario to the rate in which material is deposited:
\[ C = A \times t \label{20.9.14}\]
C= total charge (coulombs), A= current (amps), t= time (seconds)
\[\textrm{n}=\dfrac{\textrm{I x t}}{\textrm{F}}\]
I= current (amps), t= time (seconds), F= Faraday’s constant (9.65x104 C/mole), n= # of moles of e-'s
Since we want to know the mass of PbO2, we will use the equation that is in terms of moles since we will be able to convert moles to mass; \(\textrm{n}=\dfrac{\textrm{I x t}}{\textrm{F}}\)
Now for solving this basic math problem (with some stoichiometric conversions thrown in)...
1) Let's look at what we have given to us from the original problem:
I= 5.0 A, t= 2.0 h (this will need to be converted into seconds), F= Faraday's Constant (always given!)
What do we not know? ... the number of moles of electrons of PbO2 that is being deposited. So we shall now embark on finding this.
2) Convert anything that needs to be changed into the correct units and proceed to plug everything into our formula:
\(\textrm{time}=\textrm{(2.0 h)(3600 s/h)}=\textrm{7200 seconds} \)
... and now we're good to "plug and chug"
\[\textrm{moles e}^-=\dfrac{\textrm{(5.0 A)(7200 sec)}}{\textrm{96,486 C/mol}}=3.73\times10^{-1}\textrm{ mol e}^-\]
3) We're almost done! Now we just need to convert moles of electrons into grams since the original problem asks for the mass of PbO2 deposited:
\(\textrm{mass in g}=\textrm{(0.373 mol)(239.198 g/mol)}=\textrm{89.247 g} \)
So our final answer is 89.247 g of PbO2 deposited, in other words, 89.247 g of PbO2 reduced from the original mass.
Q14.6.7
Above approximately 500 K, the reaction between NO2 and CO to produce CO2 and NO follows the second-order rate law Δ[CO2]/Δt = k[NO2][CO]. At lower temperatures, however, the rate law is Δ[CO2]/Δt = k′[NO2]2, for which it is known that NO3 is an intermediate in the mechanism. Propose a complete low-temperature mechanism for the reaction based on this rate law. Which step is the slowest?
Given that NO3 is an intermediate in the low temperature reaction mechanism, we automatically know two things: 1) NO3 won't show up in the final overall reaction, and thus, 2) NO3 will be in the products of the first reaction of the mechanism and in the reactants of the second reaction. We can also assume that since we're given an intermediate from the problem, this is the only intermediate (so we won't have to dream up any other compounds that might exist in the series of reactions.) These things being said, the lower temperature reaction mechanism will look like this:
\[2NO_2(g) \rightarrow NO_3(g) + NO(g) \tag{1}\]
\[CO(g) + NO_3(g) \rightarrow CO_2(g) + NO_2(g) \tag{2}\]
And the overall reaction will look like this (notice how NO3 is not present):
\[CO(g) + NO_2(g) \rightarrow CO_2(g) + NO(g) \tag{overall reaction}\]
Now that we have the reaction mechanism written out, we can go about determining which step is the slowest. It would be pretty tricky to do this if we weren't given any further information, however, we know two more things: 1) the high-temperature mechanism's rate = k[NO2][CO] meaning that it took place in one step (given that the overall reaction is also equal to this rate) and 2) the low-temperature mechanism's rate = k′[NO2]2. These two things being said, we've both confirmed that our proposed low-temperature reaction mechanism is in fact two steps, and that we have a means to find which step is slower.
By using the "guess-and-check" method we can label each step reaction one at a time as the "slow reaction" and see if the rate matches up with the rate given to us.
Let's first try the 2nd reaction. (see above)
By using rate laws we can determine that the rate of the reaction must be in terms of its reactants, which follows:
\[\textrm{k}\textrm{[CO][NO}_3\textrm{]}\]
... But wait! We can't have the overall reaction rate in terms of an intermediate.
By looking at the 1st reaction, we can determine that we can sub in "[NO2] /[NO]" for "[NO3]" since by writing the full reaction rate of the first step and solving for [NO3] this is equivalent. So, we now have the overall rate of the mechanism as the following given the 2nd reaction is the "slow reaction:"
\[\textrm{k}\dfrac{\textrm{[CO][NO}_2\textrm{]}^2}{\textrm{[NO}\textrm{]}}\]
Note that NO2 is raised to the second power to account for the stoichiometry of the balanced reaction.
So clearly the 2nd reaction isn't the slow reaction since the rate is not equivalent to what we were given!
Let's check the rate of the 1st reaction now...
\[\textrm{k}\textrm{[NO}_2\textrm{]}^2\]
What do you know... the rates are equal! (yes, I made you go through both equations on purpose... a little extra practice never hurt anybody)
We have now confirmed that the 1st reaction is the slow reaction equation, since its rate is equivalent to the overall reaction rate.
For more on reaction rate laws, see Section 14.3
(End of Extra Credit Phase I)
_________________________________________________________________________________________________________________________________________
(Start of Extra Credit Phase II)
Q 17.2.5
Identify the species oxidized, species reduced, and the oxidizing agent and reducing agent for all the reactions in the previous problem.
"Previous Problem" Equations will be shown for each lettered portion of this question.
A) \(\textrm{Al(s)} +\textrm{Zr}^{+4}\textrm{(aq)}\rightarrow \textrm{Al}^{+3}\textrm{(aq)} +\textrm{Zr(s)}\)
In order to determine the four terms stated above when applying them to our given chemical reaction, we should first be aware of what each of these terms mean.
Oxidation - a chemical species loses electrons (e-) and (and charge), so its charge becomes more positive
Reduction - a chemical species gains electrons (e-) and thus, "gains" more negative charge
Oxidizing Agent - this name is super deceiving because an "oxidizing" agent refers to the species in a given reaction that is being reduced (and thus does the oxidizing of the other species by "accepting" electrons)
Reducing Agent - the exact opposite of an oxidizing agent; refers to the species in a given reaction that is being oxidized (and thus does the reducing of the other species by "giving" electrons)
Refer to Section 20.1 for more on oxidation-reduction reactions
Now that we have a general idea of what the question is asking us of, we shall go about finding these terms. The first thing I usually look at are the charges of the species on the reactants side of the reaction (since we are analyzing the forward reaction.) In our given equation, we have \(\textrm{Al(s)}\) and \(\textrm{Zr}^{+4}\textrm{(aq)}\) as the reactants. Now this is fine and dandy, but the reactants don't mean anything until we compare them to the products, \(\textrm{Al}^{+3}\textrm{(aq)}\) and \(\textrm{Zr(s)}\). When comparing the reactants and products, which species acquires a more positive charge, in other words, which species is oxidzed? Also, which species acquires a more negative charge - which species is reduced? (Al(s) and Zr4+(aq) respectively!)
So, based on the above definitions of oxidation and reduction in correspondence to oxidizing and reducing agents, Al(s) is being oxidized and serves as the reducing agent since it "gives up" electrons as depicted by its more positive charge, and Zr4+(aq) is being reduced and serves as the oxidizing agent since it "accepts" electrons as depicted by its more negative charge.
Now that we've done one, the next three should be cake.
B) \(\textrm{Ag}^{+}\textrm{(aq)} +\textrm{NO(g)}\rightarrow \textrm{Ag(s)} +\textrm{NO}_3^{-}\textrm{(aq)}\)
Reactants: \(\textrm{Ag}^{+}\textrm{(aq)}\), \(\textrm{NO(g)}\)
Products: \(\textrm{Ag(s)}\), \(\textrm{NO}_3^{-}\textrm{(aq)}\)
BUT WAIT... this one isn't as straightforward since we have multi-element compounds... or is it?
Ag+(s) is "straightforward" since by glancing at the reaction, we can see that it gains one electron based on its charge; +1 to 0.
NO(g) isn't as easy, though it's not hard.
First, we need to go about isolating each element. Based on the tendencies of oxygen, we can assume that it has a -2 charge. Given the compound has a charge of 0 and oxygen's charge is -2, we can use basic algebra to figure out the charge of N:
\[\textrm{charge of N} + \textrm{-2} = \textrm{0}\] \[\textrm{charge of N} = \textrm{+2}\]
Next, we need to compare the charges of each element on the reactant side to their charges on the product side. Lucky for us, it is safe to assume that Oxygen will continue to hold its -2 charge, and thus, we can solve for the charge of N again given the NO compound now holds a - charge.
\[\textrm{charge of N} + \textrm{-6} = \textrm{-1}\] \[\textrm{charge of N} = \textrm{+5}\]
Now that we've isolated the charges of each elemental species, we can see that Oxygen holds a constant charge of -2 (thus is neither a reducing nor oxidizing agent) and that Nitrogen goes from a +2 charge to +5.
Given that Ag+(s) goes from a charge of +1 to 0, we can conclude that Ag+(s) is being reduced, and is thus the oxidizing agent as it "accepts" an electron.
In addition, since N goes from a charge of +2 to +5, we can conclude that N is being oxidized, and is thus the reducing agent as it "donates" electrons.
C) \(\textrm{SiO}_3^{-2}\textrm{(aq)} +\textrm{Mg(s)}\rightarrow \textrm{Si(s)} +\textrm{Mg(OH)}_2\textrm{(s)}\)
Again, we need to go about isolating each element. We'll start with Silicon.
Based on the tendencies of oxygen, we can assume that it has a -2 charge. Given the compound has a charge of -2 and that oxygen's charge is -2 times 3 (since we have three oxygens,) we can use basic algebra to figure out the charge of Si:
\[\textrm{charge of Si} + \textrm{-6} = \textrm{-2}\] \[\textrm{charge of Si} = \textrm{+4}\]
(this charge calculation is incorrect in Uyen Ngo's extra credit phase I)
Silicon on the product side is easy; Si(s) has a charge of 0.
Onto Magnesium. In Mg's case, the reactant side is easy; the charge of Mg(s) = 0 (the charge of an element on its own in solid form will typically always be 0 unless otherwise labelled.) Next, we need to compare this charge to the product side. We can assume that OH (hyrdoxide) carries a -1 charge (-1 times 2 since we have two hydroxides,) and thus, we can solve for the charge of Mg given the Mg(OH)2 compound holds a 0 charge.
\[\textrm{charge of Mg} + \textrm{-2} = \textrm{0}\] \[\textrm{charge of Mg} = \textrm{+2}\]
Given that Si goes from a charge of +4 to 0, we can conclude that Si is being reduced, and is thus the oxidizing agent as it "accepts" an electron.
In addition, since Mg goes from a charge of 0 to +2, we can conclude that Mg is being oxidized, and is thus the reducing agent as it "donates" electrons.
D) \(\textrm{ClO}_3^{-}\textrm{(aq)} +\textrm{MnO}_2\textrm{(s)}\rightarrow \textrm{Cl}^{-}\textrm{(aq)} +\textrm{MnO}_4^{-}\textrm{(aq)}\)
You know it, we'll go about isolating each element. We'll start with Chlorine.
Based on the tendencies of oxygen, we can assume that it has a -2 charge. Given the compound has a charge of -1 and that oxygen's charge is -2 times 3 (since we have three oxygens,) we can use basic algebra to figure out the charge of Cl:
\[\textrm{charge of Cl} + \textrm{-6} = \textrm{-1}\] \[\textrm{charge of Cl} = \textrm{+5}\]
Chlorine on the product side is easy since the charge is given to us; Cl-(aq) has a charge of -1.
Onto Manganese.
Given the compound in which Mn is present in on the reactant side has a charge of 0 and that oxygen's charge is -2 times 2 (since we have two oxygens,) we can use basic algebra to figure out the charge of Mn:
\[\textrm{charge of Mn} + \textrm{-4} = \textrm{0}\] \[\textrm{charge of Mn} = \textrm{+4}\]
Given the compound in which Mn is present in on the product side has a charge of -1 and that oxygen's charge is -2 times 4 (since we have four oxygens,) we can use basic algebra to figure out the charge of Mn:
\[\textrm{charge of Mn} + \textrm{-8} = \textrm{-1}\] \[\textrm{charge of Mn} = \textrm{+7}\]
Given that Cl goes from a charge of +5 to -1, we can conclude that Si is being reduced, and is thus the oxidizing agent as it "accepts" an electron.
In addition, since Mn goes from a charge of +4 to +7, we can conclude that Mg is being oxidized, and is thus the reducing agent as it "donates" electrons.
Q19.1.14
A 2.5624-g sample of a pure solid alkali metal chloride is dissolved in water and treated with excess silver nitrate. The resulting precipitate, filtered and dried, weighs 3.03707 g. What was the percent by mass of chloride ion in the original compound? What is the identity of the salt?
We don't know the full identity of the compound being dissolved into water, so we will let x = the unknown alkali metal in the "solid alkali metal chloride" compound. We can write it this way in the following balanced equation of the reaction:
\[\textrm{XCl(aq)} +\textrm{AgNO}_3\textrm{(aq)}\rightarrow \textrm{XNO}_{3}\textrm{(aq)} +\textrm{AgCl(s)}\]
Next, we'll determine the moles of AgCl present in the reaction since 1) the mass of the precipitate is given to us and 2) this value can help us determine the moles of alkali metal chloride compound present. Given the mass of AgCl is 3.03707g in the problem and the molecular mass of AgCl per mole is 143.32g, we can solve for how many moles of AgCl is in the reaction:
\[\dfrac{\textrm{3.03707 g}}{\textrm{143.32 g/mol}} = \textrm{0.0211 mol}\]
And since the molar ratio of AgCl to XCl is 1:1, we can conclude that there are 0.0211 mol of XCl present.
Given we have 0.0211 mol of XCl, and thus, of Cl, we can find out the mass of Cl that we have in XCl:
\[\textrm{(0.0211 mol)(35.5 g/mol)} = \textrm{0.7490g}\]
And the mass percentage of Cl in the alkali metal compound is:
\[\dfrac{\textrm{0.7490}}{\textrm{2.5624}} \textrm{ x 100} = \textrm{29.23%}\]
Now that we have the mass of Cl and its mass percentage, we can move on to determining the unknown alkali metal. We can do this by determining its mass and comparing its molar mass to known elements on the periodic table. To find out the mass of the unknown alkali metal in the compound, given XCl weights 2.562g, we can subtract the overall compound mass by the mass of Cl:
\[\textrm{(2.562 g - 0.7490 g)} = \textrm{1.8134g}\]
And now, since we have the mass of the unknown metal, we can calculate its molar mass by dividing the above value by the number of moles of XCl that it present in the reaction, 0.0211.
\[\textrm{Atomic Mass of Metal} = \dfrac{\textrm{Molar Mass of Metal}}{\textrm{Total Mass of AgCl}} = \dfrac{\textrm{1.8134}}{\textrm{0.0211}} = 85.943 g/mol\]
And since the molar mass of Rubidium (Rb), an alkali metal, on the periodic table is 85.468 g, we can conclude from our calculations that Rb is the identity of the unknown metal, and thus, the identity of the salt is RbCl.
Q19.3.6
How many unpaired electrons are present in each of the following?
- [CoF6]-2 (high spin)
- [Mn(CN)6]-3 (low spin)
- [Mn(CN)6]-4 (low spin)
- [MnCl6]-4 (high spin)
- [RhCl6]-3 (low spin)
To find unpaired electrons, we first need to be able to find out how many electrons we have to begin with for each compound. For any transition metal compound, we can do this by finding the electron configuration for the central transition metal. To do this, we must find out its charge. Let's use the first compound, [CoF6]-3 , as an example for this process.
1. [CoF6]-3 (high spin)
We can find out Cobalt's charge by subtracting the charge of the ligand in the compound, F- (-1 times 6, since we have 6 Fluorine atoms,) from the overall charge of the compound, -3. \[\textrm{charge of Co} + \textrm{-6} = \textrm{-6}\] \[\textrm{charge of Co} = \textrm{+3}\] So our transition metal is now confirmed to be Cobalt(III). In other words, Cobalt has a charge of +3. Now we can draw the following conclusions about the number of electrons in the d-orbital (which is what we will always pay attention to when determining unpaired electrons in 1st row transition metal compounds):
1) Cobalt has no electrons in its S shell (those two electrons are not present due to the Co+3 ion.
2) Cobalt has one less electron than "normal" in its d-orbital since it "loses" a third electron in addition to the first two that it "lost."
So, by looking at the periodic table, we can see that Cobalt has 7 - 1 = 6 electrons in its d-orbital.
Now, given that [CoFg]-3 is high spin, we will distribute the electrons throughout both rows of the crystal field splitting diagram then continue to fill the bottom row once both the top and bottom are filled with one electron for each slot:

Cyrstal splitting diagrams courtesy of Uyen Ngo
By looking at this diagram, we can conclude that there are 4 unpaired electrons in the [CoF6]-3 compound.
See Section 21.7 for more on Crystal Field Splitting and depicting the energy levels of electrons
2. [Mn(CN)6]-3 (low spin)
NOTE: CN has a -1 charge. Since we have 6 CN's, this ligand will carry a -6 charge as depicted in the algebra for obtaining Mn's charge below: \[\textrm{charge of Mn} + \textrm{-6} = \textrm{-3}\] \[\textrm{charge of Mn} = \textrm{+3}\]
Given Mn has a +3 charge, we can conclude that there are 4 electrons in its d orbital. Since [Mn(CN)6]-3 is low spin, the energy diagram below shows how its 4 electrons will be arranged with crystal field splitting:

There are 2 unpaired electrons in the [Co(CN)6]-3 compound.
3. [Mn(CN)6]-4 (low spin)
\[\textrm{charge of Mn} + \textrm{-6} = \textrm{-4}\] \[\textrm{charge of Mn} = \textrm{+2}\]
Given Mn has a +2 charge, we can conclude that there are 5 electrons in its d orbital. Since [Mn(CN)6]-3 is low spin, the energy diagram below shows how its 5 electrons will be arranged with crystal field splitting:
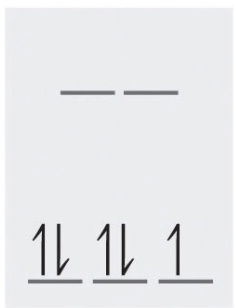
There is 1 unpaired electron in the [Mn(CN)6]-4 compound.
4. [MnCl6]-4 (high spin)
\[\textrm{charge of Mn} + \textrm{-6} = \textrm{-4}\] \[\textrm{charge of Mn} = \textrm{+2}\]
Given Mn has a +2 charge, we can conclude that there are 5 electrons in its d orbital. Since [Mn(CN)6]-4 is high spin, the energy diagram below shows how its 5 electrons will be arranged with crystal field splitting:

There are 0 unpaired electrons in the [Mn(Cl)6]-4 compound.
5. [RhCl6]-3 (low spin)
\[\textrm{charge of Rh} + \textrm{-6} = \textrm{-3}\] \[\textrm{charge of Mn} = \textrm{+3}\]
Given Rh has a +3 charge, we can conclude that there are 6 electrons in its d orbital. Since [Rh(Cl)6]-3 is low spin, the energy diagram below shows how its 6 electrons will be arranged with crystal field splitting:

There are 0 unpaired electrons in the [Rh(Cl)6]-3 compound.
Q12.4.6
What is the half-life for the first-order decay of phosphorus-32? \(\ce{(^{32}_{15}P \rightarrow ^{32}_{16}S + e- )}\) The rate constant for the decay is 4.85 × 10−2 day−1.
The formula that relates the decay constant to a first order half-life reaction follows:
\[\textrm{T}_{1/2} =\dfrac{\textrm{ln2}}{\textrm{λ}}\]
With this formula, it is now very easy to solve this problem. Since it is given to us that λ = 4.85 × 10−2 day−1, we can plug this value into the formula and proceed to solve for a time value:
\[\textrm{T}_{1/2} =\dfrac{\textrm{ln2}}{\textrm{λ}} = \dfrac{\textrm{ln2}}{\textrm{4.85 x 10}^{-2}\textrm{day}^{-1}} = \textrm{14.3 days}\]
So, the half-life is 14.3 days.
See the article, "Half-Lives," for more information on half-life and decay constants
Q21.2.1
Write the following isotopes in hyphenated form (e.g., “carbon-14”)
- \(\ce{^{24}_{11}Na}\)
- \(\ce{^{29}_{13}Al}\)
- \(\ce{^{73}_{36}Kr}\)
- \(\ce{^{194}_{77}Ir}\)
When writing isotopes in hyphenated form, we can follow three simple steps:
1) Write the name of the element
2) After the element's name, place a hyphen
3) After the hyphen, write down the mass # of the given isotope (this number will be equal to the isotopes's number of protons + neutrons)
... and voila, you have an isotope written in hyphenated form!
Let's apply the above steps to our given problem.
1. Na = Sodium; add a hyphen; mass # = 24: Sodium-24
2. Al = Aluminum; add a hyphen; mass # = 29: Aluminum-29
3. Kr = Krypton; add a hyphen; mass # = 73: Krypton-73
4. Ir = Iridium; add a hyphen; mass # = 194: Iridium-194
Q21.5.4
Cite the conditions necessary for a nuclear chain reaction to take place. Explain how it can be controlled to produce energy, but not produce an explosion.
First of all, what's a "nuclear chain reaction?" It's exactly what it sounds like: a series of nuclear reactions, each reaction caused by the reaction prior to it.
Typically nuclear chain reactions occur as a result of the nuclear fission of large elements (such as U-235). In the case of the nuclear fission of Uranium, it splits into two or three neutrons, which as one can imagine, produces a tremendous amount of energy (about 2.5 million times as much energy as burning 1 kg of coal to be exact!)
See Section 5.8 for more specifics on nuclear fission.
Now what happens to the by-products of the nuclear fission of Uranium? They are very likely to cause the fission of other Uranium atoms, (which in turn cause the fission of more Uranium atoms...) and Viola! We've got a nuclear chain reaction.
So back to the original question...
The "conditions" necessary for a nuclear chain reaction to take place require that an atom 1) undergoes fission (or fusion) and 2) in turn causes other atoms to undergo fission. Other factors such as high temperature and whether or not the nuclei indeed collided also play a role in setting off nuclear chain reactions.
The next portion of our question asks how a nuclear chain reaction can be controlled to produce energy (and to not produce an explosion which is very much preferable.)
The simple answer to this - with a nuclear reactor.
A nuclear reaction is able to prevent explosions by separating fissionable nuclear material (and essentially preventing the nuclear particles from continually reacting with themselves in a manner that is out of control.) In addition to preventing disaster, nuclear reactors are also able to generate power by trapping fission energy as thermal energy, which goes on to boil water and produce steam. The steam turns a turbine, which powers a generator that produces electricity!
Q20.4.2
List two factors that affect the measured potential of an electrochemical cell and explain their impact on the measurements.
First of all, let's get a general gist of what main three values are present in an electrochemical cell:
E°cell - "cell potential;" the amount of voltage that exists between the two half cells of a battery
ΔG° - the measure of the maximum amount of work that can be performed during a chemical process
K - represents the "equilibrium constant" of a given reaction: the state in which the reactants and products have no net change over time
In determining factors that affect the measured potential of an electrochemical cell, we can speak abstractly. However, the most concrete evidence to help in proving what variables affect cell potential comes from the formulas that relate these variables:

(From Section 19.3) The Relationships among Criteria for Thermodynamic Spontaneity. The three properties of a system that can be used to predict the spontaneity of a redox reaction under standard conditions are K, ΔG°, and E°cell. If we know the value of one of these quantities, then these relationships enable us to calculate the value of the other two. The signs of ΔG° and E°cell and the magnitude of K determine the direction of spontaneous reaction under standard conditions.
When looking at the "Big Triangle" (shown above), we can draw the following conclusions regarding which factors affect the measured potential of an electrochemical cell...
1) Temperature is one thing that affects the measured potential of an electrochemical cell. As one can see in the equation relating E0 cell to the equilibrium constant K (shown below), an increase in temperature makes a reaction at a cell more favorable (since the overall E0 cell will become more positive) while a decrease in temperature causes the reaction to be less favorable.
\[\textrm{E}^{0}_{\textrm{cell}} = \dfrac{\textrm{RT}}{\textrm{nF}}\textrm{lnK}\]
2) Concentration is another variable that contributes to cell potential. Once again, we can look to formula of the relationship between E0cell and K to see this. K refers to the concentrations of the compounds in a given reaction, and thus, concentration plays a role in the value of E0cell . Another way to write this relationship in terms of any condition (non-standard conditions) looks like this:
\[\textrm{E}_{\textrm{cell}} = \textrm{E}^{0}_{\textrm{cell}} -\dfrac{\textrm{RT}}{\textrm{nF}}\textrm{lnQ}\]
Q represents the reaction quotient, which can be written like this for the sample reaction, \(\textrm{A} \rightarrow \textrm{B + C}\):
\[\textrm{Q} = \dfrac{\textrm{[B}\textrm{]}\textrm{[C}\textrm{]}}{\textrm{[A}\textrm{]}}\]
Given that Q is made up of the concentrations in a given reaction, we can conclude that these concentrations in fact affect Ecell given that Q affects Ecell .
Though this question does not ask for a third factor, it's easy to prove that pressure is also a factor by the reaction quotient formula:
\[\textrm{Q} = \dfrac{\textrm{[B}\textrm{]}\textrm{[C}\textrm{]}}{\textrm{[A}\textrm{]}}\]
which is also equal to...
\[\textrm{Q} = \dfrac{\textrm{P}_{\textrm{B}}\textrm{P}_{\textrm{C}}}{\textrm{P}_{\textrm{A}}}\]
where P = pressure, so yes, pressure also affects Ecell.
(Uyen Ngo didn't list a second factor in extra credit phase I)
Q20.5.31
The silver–silver bromide electrode has a standard potential of 0.07133 V. What is Ksp of AgBr?
From the problem, standard potential is given and we are out to solve for Ksp . So we need a formula that connects both of these terms.
Looking back at the Big Triangle from Q20.4.2 again, the Nernst equation follows:
\[\textrm{E}^{0}_{\textrm{cell}} =\dfrac{\textrm{RT}}{\textrm{nF}}\textrm{lnK}\]
However, its hard to see at first glance how the Nernst equation relates E0cell to Ksp. So, let's manipulate this formula to be in terms of Ksp.
\[\textrm{lnK}_{sp} =\dfrac{\textrm{nFE}^{0}}{\textrm{RT}}\]
Now we can plug in the values that are given to us and solve for our unknown, Ksp ...
Given:
E0cell = 0.07133V,
n = 1 mol,
F = 96485 C,
R = 8.314 Jmol-1K-1
T = 273K
Now plug and chug ...
\[\textrm{lnK}_{sp} =\dfrac{\textrm{nFE}^{0}}{\textrm{RT}} = \dfrac{\textrm{(1)(96485C)(0.07133 V)}}{\textrm{(8.314 J/mol/K)(273 K)}} = \textrm{3.03}\tag{1}\]
\[\textrm{e}^{\textrm{lnK}_{sp}} = \textrm{e}^{3.03}\tag{2}\]
\[\textrm{K}_{sp} = \textrm{2.07 x 10}^{1}\tag{3}\]
So, the Ksp of AgBr is 2.07 x 101
(*NOTE: Ksp doesn't have any units!)
Click here for more on exclusively the Nernst equation

