8.2: Collision Theory and Reaction Mechanisms
- Page ID
- 431440
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)By the end of this section, you will be able to:
- Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates
- Define the concepts of activation energy and transition state
- Distinguish net reactions from elementary reactions (steps)
- Identify the molecularity of elementary reactions
- Write a balanced chemical equation for a process given its reaction mechanism
Introduction to Collision Theory
We should not be surprised that atoms, molecules, or ions must collide before they can react with each other. Atoms must be close together to form chemical bonds. This simple premise is the basis for a very powerful theory that explains many observations regarding chemical kinetics, including factors affecting reaction rates.
Collision theory is based on the following postulates:
- The rate of a reaction is proportional to the rate of reactant collisions.
- The reacting species must collide in an orientation that allows contact between the atoms that will become bonded together in the product.
- The collision must occur with adequate energy to permit mutual penetration of the reacting species’ valence shells so that the electrons can rearrange and form new bonds (and new chemical species).
The first postulate for collision theory explains why most reaction rates increase as concentrations increase. With an increase in the concentration of any reacting substance, the chances for collisions between molecules are increased because there are more molecules per unit of volume. More collisions mean a faster reaction rate, assuming the energy of the collisions is adequate.
To explain the second and third postulates, the orientation and energy of collisions, consider the reaction of carbon monoxide with oxygen:
Carbon monoxide is a pollutant produced by the combustion of hydrocarbon fuels. To reduce this pollutant, automobiles have catalytic converters that use a catalyst to carry out this reaction. If carbon monoxide and oxygen are present in sufficient amounts, the reaction will occur at high temperature and pressure. The first step in the gas-phase reaction between carbon monoxide and oxygen is a collision between the two molecules:
CO(g) + O2(g) ⟶ CO2(g) + O(g)
Although there are many different possible orientations the two molecules can have relative to each other, consider the two presented in Figure \(\PageIndex{1}\). In the first case, the oxygen side of the carbon monoxide molecule collides with the oxygen molecule. In the second case, the carbon side of the carbon monoxide molecule collides with the oxygen molecule. The second case is clearly more likely to result in the formation of carbon dioxide, which has a central carbon atom bonded to two oxygen atoms This shows how important the orientation of the collision is in terms of creating the desired product of the reaction.
If the collision does take place with the correct orientation, there is still no guarantee that the reaction will proceed to form carbon dioxide. In addition to a proper orientation, the collision must also occur with sufficient energy to result in product formation. When reactant species collide with both proper orientation and adequate energy, they combine to form an unstable species called an activated complex or a transition state. These species are very short lived and usually undetectable by most analytical instruments. In some cases, sophisticated spectral measurements have been used to observe transition states.
Activation Energy
The minimum energy necessary to form a product during a collision between reactants is called the activation energy (Ea). The magnitude of the activation energy compared to the kinetic energy from colliding reactant molecules is an important factor determining the rate of a chemical reaction. If the activation energy is much larger than the average kinetic energy of the collisions, the reaction occurs slowly since only a few molecules move fast enough to have enough energy to react. If the activation energy is much smaller than the average kinetic energy of the molecules, a large fraction of molecules have enough kinetic energy to react when they collide.
Figure \(\PageIndex{2}\) shows how the energy of a chemical system changes as it undergoes a reaction converting reactants to products according to the equation
A + B ⟶ C + D
These reaction diagrams are widely used in chemical kinetics to illustrate various properties of the reaction of interest. Viewing the diagram from left to right, the system initially comprises reactants only, A + B. Reactant molecules with sufficient energy can collide to form a high-energy activated complex or transition state. The unstable transition state can then subsequently decay to yield stable products, C + D. The diagram depicts the reaction's activation energy, Ea, as the energy difference between the reactants and the transition state. Using a specific energy, the enthalpy (see chapter on thermochemistry), the enthalpy change of the reaction, ΔH, is estimated as the energy difference between the reactants and products. In this case, the reaction is exothermic (ΔH < 0) since it yields a decrease in system enthalpy.
Boltzman Distribution
The energy required to overcome the activation energy is provided by the kinetic energy of the molecules when they collide. As a result, the rate of the reaction - how many molecules can overcome the activation energy barrier - depends upon the temperature. The boltzmann distribution shown in Figure \(\PageIndex{3a}\) describes the number of molecules as a function of kinetic energy at a specific temperature. When the temperature changes as shown in Figure \(\PageIndex{3b}\) the distribution changes, so the number of molecules with enough kinetic energy to overcome the activation energy barrier changes.
Reaction Mechanisms
Chemical reactions very often occur in a step-wise fashion, involving two or more distinct reactions taking place in sequence. A balanced equation indicates what is reacting and what is produced, but it reveals no details about how the reaction actually takes place. The reaction mechanism (or reaction path) provides details regarding the precise, step-by-step process by which a reaction occurs.
The decomposition of ozone, for example, appears to follow a mechanism with two steps:
\( \mathrm{O}_{3 (g)} \longrightarrow \mathrm{O}_{2(g)}+\mathrm{O} \)
\( \mathrm{O} + \mathrm{O}_{3 (g)} \longrightarrow \mathrm{2O}_{2(g)} \)
Each of the steps in a reaction mechanism is an elementary reaction. These elementary reactions occur precisely as represented in the step equations, and they must sum to yield the balanced chemical equation representing the overall reaction:
\( \mathrm{2 O}_{3 (g)} \longrightarrow \mathrm{3O}_{2(g)} \)
Notice that the oxygen atom produced in the first step of this mechanism is consumed in the second step and therefore does not appear as a product in the overall reaction. Species that are produced in one step and consumed in a subsequent step are called intermediates. Intermediates are different from transition states because the intermediates are longer lived and can be detected in many reactions. A transition state is at the top of the reaction energy diagram - as shown in Figure \(\PageIndex{2}\). Figure \(\PageIndex{4}\) shows the O3 starting material, the first transition state is the excited state ozone molecule, O3*, the reaction intermediate O in the valley between the two transition state peaks, the second transition state O-O3*, and the final products O2. T
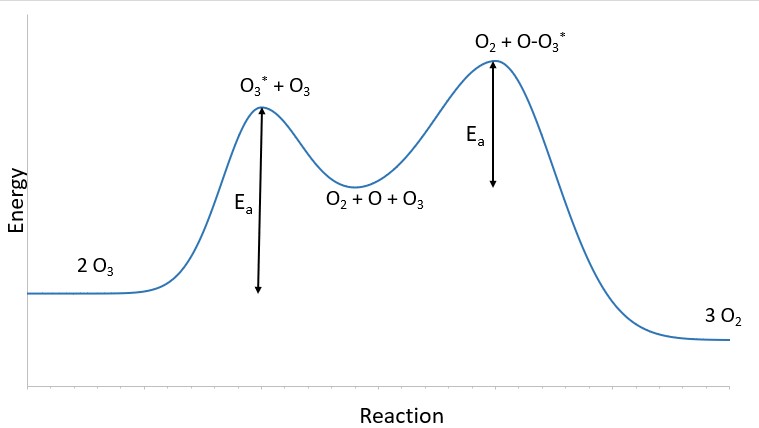
While the overall reaction equation for the decomposition of ozone indicates that two molecules of ozone react to give three molecules of oxygen, the mechanism of the reaction does not involve the direct collision and reaction of two ozone molecules. Instead, one O3 decomposes to yield O2 and an oxygen atom, and a second O3 molecule subsequently reacts with the oxygen atom to yield two additional O2 molecules.
Unlike balanced equations representing an overall reaction, the equations for elementary reactions are explicit representations of the chemical change taking place. The reactant(s) in an elementary reaction’s equation undergo only the bond-breaking and/or making events depicted to yield the product(s).
Unimolecular Elementary Reactions
The molecularity of an elementary reaction is the number of reactant species (atoms, molecules, or ions). For example, a unimolecular reaction involves the reaction of a single reactant species to produce one or more molecules of product:
\( \mathrm{A} \longrightarrow \mathrm{products} \)
The rate law for a unimolecular reaction is first order because it only depends upon the amount of one reaction:
A unimolecular reaction may be one of several elementary reactions in a complex mechanism. For example, the reaction:
O3 (g) -> O2 (g) + O
illustrates a unimolecular elementary reaction that occurs as one part of a two-step reaction mechanism as described above. However, in some cases the overall reaction is also an elementary reaction. For some unimolecular reactions are the only step of a single-step reaction mechanism. For example, the gas-phase decomposition of cyclobutane, C4H8, to ethylene, C2H4, is represented by the chemical equation in Figure \(\PageIndex{4}\):
Figure \(\PageIndex{5}\). Decomposition of cyclobutane.
This equation represents the overall reaction observed, and it might also represent a legitimate unimolecular elementary reaction. The rate law predicted from this equation, assuming it is an elementary reaction, turns out to be the same as the rate law derived experimentally for the overall reaction, namely, one showing first-order behavior:
rate = k[C4H8]
This agreement between observed and predicted rate laws is interpreted to mean that the proposed unimolecular, single-step process is a reasonable mechanism for the butadiene reaction.
Bimolecular Elementary Reactions
A bimolecular reaction involves two reactant species, for example:
\( \mathrm{A}+\mathrm{A} \longrightarrow \mathrm{products} \)
OR
\( \mathrm{A}+\mathrm{B} \longrightarrow \mathrm{products} \)
For the first type, in which the two reactant molecules are different, the rate law is first-order in A and first order in B (second-order overall):
\( \mathrm{RATE}= \mathrm{k[A][B]} \)
For the second type, in which two identical molecules collide and react, the rate law is second order in A:
\( \mathrm{RATE}=\mathrm{k[A][A]}=\mathrm{k[A]^2} \)
Bimolecular elementary reactions may also be involved as steps in a multistep reaction mechanism. The reaction of atomic oxygen with ozone is the second step of the two-step ozone decomposition mechanism discussed earlier in this section.
Relating Reaction Mechanisms to Rate Laws
It’s often the case that one step in a multistep reaction mechanism has a much higher activation energy, so it is significantly slower than the others. Because a reaction cannot proceed faster than its slowest step, this step will limit the rate at which the overall reaction occurs. The slowest step is therefore called the rate-limiting step (or rate-determining step) of the reaction. Figure \(\PageIndex{7}\) shows a cattle chute, which is the rate-determining step for moving cattle between two pens.
As described earlier, rate laws may be derived directly from the chemical equations for elementary reactions. However, most chemical reactions are not elementary reactions so and they are often the result of several elementary reaction steps. Together these steps make up the reaction mechanism. In every case, the rate law must be determined from experimental data and the reaction mechanism subsequently deduced from the rate law (and sometimes from other data). The reaction of NO2 and CO provides an illustrative example:
NO2(g)+CO(g)⟶CO2(g)+NO(g)
For temperatures above 225 °C, the rate law is:
rate = k [NO2][CO]
The reaction is first order with respect to NO2 and first-order with respect to CO. This is consistent with a single-step bimolecular mechanism and it is possible that this is the mechanism for this reaction at high temperatures.
At temperatures below 225 °C, the reaction is described by a rate law that is second order with respect to NO2:
rate=k[NO2]2
This rate law is not consistent with the single-step mechanism, but is consistent with the following two-step mechanism:
NO2(g)+NO2(g)⟶NO3(g)+NO(g) (slow)
NO3(g)+CO(g)⟶NO2(g)+CO2(g) (fast)
The rate-determining (slower) step gives a rate law showing second-order dependence on the NO2 concentration, and the sum of the two equations gives the net overall reaction. This shows the reaction using different mechanisms depending upon the temperature. The two reaction mechanisms are shown in Figure \(\PageIndex{8}\).

In general, when the rate-determining (slower) step is the first step in a mechanism, the rate law for the overall reaction is the same as the rate law for this step. However, when the rate-determining step is preceded by a step involving a rapidly reversible reaction the rate law for the overall reaction may be more difficult to derive.


