LAB 3 - ALCOHOLS AND AMINES
- Page ID
- 506280
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\(\newcommand{\ket}[1]{\left| #1 \right>} \)
\( \newcommand{\bra}[1]{\left< #1 \right|} \)
\( \newcommand{\braket}[2]{\left< #1 \vphantom{#2} \right| \left. #2 \vphantom{#1} \right>} \)
\( \newcommand{\qmvec}[1]{\mathbf{\vec{#1}}} \)
\( \newcommand{\op}[1]{\hat{\mathbf{#1}}}\)
\( \newcommand{\expect}[1]{\langle #1 \rangle}\)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The purpose of this experiment is to:
- Draw structures of alcohols and amines
- Name alcohols and amines
- Explore the physical properties and reactions of alcohols and amines
- Perform chemical tests on alcohols
INTRODUCTION
Alcohols are organic compounds with -OH bonded to carbon, and amines have a nitrogen bonded to a carbon chain. Both alcohols and amines are classified as primary, secondary, or tertiary according to the definitions provided below:
Primary Alcohol: The carbon bonded to -OH is bonded to one carbon atom.
Secondary Alcohol: The carbon bonded to -OH is bonded to two carbon atoms.
Tertiary Alcohol: The carbon bonded to -OH is bonded to three carbon atoms.
Primary amine: Nitrogen is bonded to one carbon and two hydrogen atoms.
Secondary amine: Nitrogen is bonded to two carbons and one hydrogen atom.
Tertiary amine: Nitrogen is bonded to three carbons and no hydrogen atoms.
Alcohols and amines exhibit similar physical properties, including water solubility and trends in boiling points. However, they widely differ in terms of their acid-base properties. Alcohols act as weak acids, and amines are weak bases. In this experiment, you will explore the structure, nomenclature, physical properties, and acid-base properties of alcohols and amines. The pre-lab questions will help you prepare for this part of the lab.
You will also run various reactions and chemical tests with compounds containing these functional groups. Amines react with HCl to produce amine salts, as shown by the reaction below:

Amine salts are important in medicinal chemistry because they make pharmaceutical compounds more water-soluble and bioavailable.
One of the most common reactions for alcohols is their oxidation to form aldehydes, ketones, and carboxylic acids. When alcohol is reacted with sodium dichromate in sulfuric acid (chromic acid reagent), the products of oxidation are as follows:
1) Primary alcohol is oxidized to form aldehydes, which are oxidized to carboxylic acids.
2) Secondary alcohols are oxidized to form ketones.
3) Phenols (which contain -OH bonded to a benzene ring) are oxidized to quinones.
4) Tertiary alcohols do not undergo oxidation.
The chromic acid test is a chemical test used to identify certain alcohols. Consider the reaction between 2-pentanol (pentan-2-ol) and the chromic acid reagent:

The secondary alcohol is oxidized to form a ketone. In return, chromium is reduced from the +6 oxidation state (in which the solution is orange) to the +3 oxidation state (in which the solution turns blue-green). The formation of a blue-green solution is a positive test result. A negative test result would be the persistence of an orange solution.
Alcohols can also be oxidized under certain conditions with potassium permanganate, as shown by the reaction with 1-butanol.

When the primary alcohol is oxidized to carboxylic acid, the purple color of the permanganate solution disappears as manganese is reduced from +6 to +4, forming a brown solid. A positive test result for the oxidation of alcohols with potassium permanganate will be the formation of a brown solid in various colored solutions.
In addition to the chromic acid and potassium permanganate tests, you will also run the ferric chloride test, a chemical test for phenols. Phenols react with ferric chloride, forming a purple solution, a positive test result. A negative test result, indicating the absence of a phenol, would be the presence of a yellow solution, the original color of ferric chloride.
1) Always wear goggles while working with chemicals in this lab.
2) Wear gloves while working on this experiment.
3) Always handle all chemicals under a working fume hood.
4) Dispose of all waste in the appropriate waste container, which should also be kept under a fume hood at all times.
5) Thoroughly clean all glassware and your work area at the end of the experiment.
6) Before leaving the lab, wash your hands.
EQUIPMENT AND CHEMICALS NEEDED
| Chemicals | Chemicals | Equipment |
|---|---|---|
|
Ethanol |
Phenol |
6 small or medium-sized test tubes |
|
Isopropyl alcohol |
unknown alcohol |
Test tube rack |
|
T-butyl alcohol |
Aniline |
Stirring rod |
|
Cyclohexanol |
N-methylaniline |
pH paper |
|
Triethylamine |
1 M NaOH (or similar concentration) |
Watch glass |
|
2% Chromic acid reagent |
1 M HCl (or similar concentration) |
Red litmus paper |
|
Acetone |
Concentrated HCl |
Blue litmus paper |
|
1% aqueous FeCl3 |
1% aqueous potassium permanganate solution (or similar concentration) |
Halogenated and Non-halogenated waste containers |
2% Chromic acid reagent (dissolve 2 g K2Cr2O7 in 10 mL of 6 M H2SO4; carefully add H2O to make 100 mL; must be freshly prepared) will be used by the whole class. Your instructor may have 2% Chromic acid ready for testing.
EXPERIMENTAL PROCEDURE
Part A: Structures of Alcohols and Amines
a) Draw structures (Lewis, condensed, or line-bond) for the following compounds that will be used in this experiment in the data table: Ethanol, 2-propanol, 2-methyl-2-propanol, Cyclohexanol, Phenol, Aniline, N-methylaniline, Triethylamine.
b) Classify them as primary, secondary, or tertiary.
Part B: Solubility and pH of Alcohols
1) Obtain six small or medium-sized test tubes.
2) Add the following reagents to each test tube:
Test tube 1: 1 mL of ethanol and 1 mL of water.
Test tube 2: 1 mL of 2-propanol and 1 mL of water
Test tube 3: 1 mL of 2-methyl-2-propanol and 1 mL of water.
Test tube 4: 1 mL of cyclohexanol and 1 mL of water.
Test tube 5: 1 mL of phenol and 1 mL of water.
Test tube 6: 1 mL of the unknown alcohol and 1 mL of water.
3) Thoroughly mix each solution and record your observations.
4) Test the pH of each solution with pH paper. This can be done by dipping a stirring rod into the solution and touching the tip of the stirring rod to a piece of pH paper. Be sure to rinse and wipe dry the stirring rod between each solution and use only a minimal amount of pH paper.
5) To insoluble alcohols in #3 above, add drops of 1 M NaOH until the solution tests basic with red litmus paper. (Red litmus paper will turn blue.). This can be done similarly to pH paper (#4 above). Record your observations.
6) Pour the contents of each test tube into the non-halogenated organic waste container. Clean and dry the test tubes and save them for part C.
Part C: Solubility of pH of Amines
1) Obtain the three small or medium-sized test tubes.
2) Add the following reagents to each test tube:
Test tube 1: 1 mL of aniline and 1 mL of water.
Test tube 2: 1 mL of N-methylaniline and 1 mL of water.
Test tube 3: 1 mL of triethylamine and 1 mL of water.
3) Thoroughly mix each solution and record your observations.
4) Test the pH of each solution with pH paper This can be done by dipping a stirring rod into the solution and touching the tip of the stirring rod to a piece of pH paper Be sure to rinse and wipe dry the stirring rod between each solution and use only a minimal amount of pH paper.
5) To insoluble amines in #3 above, add drops of 1 M HCl until the solution tests acidic with blue litmus paper (Blue litmus paper will turn red). pH paper can be used as an alternative to blue litmus paper (#4 above). Record your observations.
6.) Pour the contents of each test tube into the halogenated organic waste container. Clean and dry the test tubes, then store them for later use.
Part D: Volatility of Phenol and Aniline
1) Obtain a watch glass. Under the hood, add five drops of phenol and five drops of aniline to separate the ends of the watch glass.
2) Record the time it takes each substance to evaporate completely.
3) Clean and dry the watch glass, and save it for part E.
Part E: Reaction of Triethylamine with Concentrated HCl
Under the hood, add one drop of triethylamine and one drop of concentrated HCl to separate ends of a watch glass. Record your observations. (This can also be run as an instructor demo.)
Part F: Oxidation of Alcohols with the Chromic Acid Reagent
1) Obtain six small or medium-sized test tubes. Under the hood, add the following reagents:
Test tube 1: 1 mL of acetone, 10 drops of ethanol, three drops of chromic acid solution.
Test tube 2: 1 mL of acetone, 10 drops of isopropyl alcohol, three drops of chromic acid solution.
Test tube 3: 1 mL of acetone, 10 drops of 2-methyl-2-propanol, and three drops of chromic acid solution.
Test tube 4: 1 mL of acetone, 10 drops of cyclohexanol, and three drops of chromic acid solution.
Test tube 5: 1 mL of acetone, 10 drops of phenol, and three drops of chromic acid solution.
Test tube 6: 1 mL of acetone, 10 drops of the unknown alcohol, three drops of chromic acid solution.
2) Thoroughly mix each solution and record your observations.
3) Pour the contents of each test tube into the non-halogenated organic waste container. Clean and rinse the test tubes before removing them to dry. Save the test tubes for part G.
Part G: Oxidation of Alcohols with Potassium Permanganate
1) Obtain six small or medium-sized test tubes. Under the hood, add the following reagents:
Test tube 1: 10 drops of ethanol and three drops of potassium permanganate solution.
Test tube 2: 10 drops of isopropyl alcohol and three drops of potassium permanganate solution.
Test tube 3: 10 drops of 2-methyl-2-propanol and three drops of potassium permanganate solution.
Test tube 4: 10 drops of cyclohexanol, three drops of potassium permanganate solution.
Test tube 5: 10 drops of phenol and three drops of potassium permanganate solution.
Test tube 6: 10 drops of unknown alcohol and three drops of potassium permanganate solution.
2) Thoroughly mix each solution, and record your initial observations.
3) For the test tubes, after 10 minutes, add two drops of 6 M HCl to the purple permanganate solution. Record your observations.
5) Pour the contents of each test tube into the non-halogenated organic waste container. Clean and rinse the test tubes before removing them to dry. Save the test tubes for part H.
Part H. Ferric Chloride Test
1) Obtain six small or medium-sized test tubes. Under the hood, add the following reagents:
Test tube 1: 5 drops of ethanol and five drops of FeCl3 solution.
Test tube 2: 5 drops of isopropyl alcohol and five drops of FeCl3 solution.
Test tube 3: 5 drops of 2-methyl-2-propanol and five drops of FeCl3 solution.
Test tube 4: 5 drops of cyclohexanol and five drops of FeCl3 solution.
Test tube 5: and five drops of phenol and five drops of FeCl3 solution.
Test tube 6: 5 drops of unknown alcohol and five drops of FeCl3 solution.
2) Thoroughly mix each solution and record your observations.
3) Pour the contents of each test tube into the halogenated organic waste container. Clean and rinse test tubes before removing them to dry.
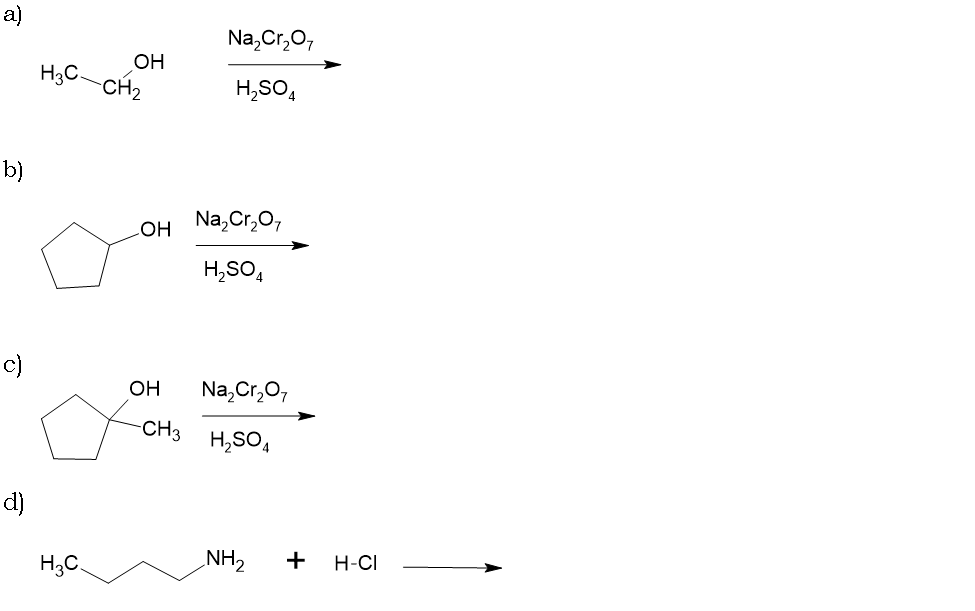
PRE-LAB QUESTIONS
Name ____________________________________
1. Provide an acceptable name for each of the following compounds.

2) Draw structures for the following compounds.
Part A: 3-pentanol
Part B: 1-methylcyclohexanol
Part C: hexan-1-ol
Part D: 3-methylphenol
Part E: 1-butanamine
Part F: N, N-dimethyl-2-pentanamine
Part G: N-ethyl-3-heptanamine
3) Identify the most important intermolecular force present in the following types of compounds:
Part A: Primary Amines
Part B: Tertiary Amines
Part C: Secondary Alcohols
Part D: Primary Alcohols
4) What is litmus paper? Explain how it can determine whether a solution is acidic or basic.
5) Predict the products of each reaction.
DATA AND OBSERVATIONS
Name _________________________Lab Partner(s) ______________________________
Part A: Structures of Alcohols and Amines
Compound |
Structure |
Classification |
|---|---|---|
|
Ethanol |
||
|
Isopropyl alcohol |
||
|
2-methyl-2-propanol |
||
|
Cyclohexanol |
||
|
Phenol |
||
|
Aniline |
||
|
N-methylaniline |
||
|
Triethylamine |
Part B: Solubility and pH of Alcohols
Test tube |
Contents |
Soluble or insoluble? |
pH |
Observations after adding NaOH (if applicable) |
|---|---|---|---|---|
|
1 |
Ethanol and Water |
|||
|
2 |
Isopropyl alcohol and Water |
|||
|
3 |
2-methyl-2-propanol and Water |
|||
|
4 |
Cyclohexane and Toluene |
|||
|
5 |
Phenol and Toluene |
|||
|
6 |
Unknown and Toluene |
Part C: Solubility and pH of Amines
Test tube |
Contents |
Soluble or insoluble? |
pH |
Observations after adding HCl (if applicable) |
|---|---|---|---|---|
|
1 |
Aniline and water |
|||
|
2 |
N-methylaniline and water |
|||
|
3 |
Triethylamine and water |
Part D: Volatility of Phenol and Aniline
Compound |
Time for Complete Evaporation |
|---|---|
|
Phenol |
|
|
Aniline |
Part E: Reaction with Triethylamine and Concentrated HCl
Record your observations here:
Write the balanced equation for the reaction:
Parts F-H: Chemical Tests for Alcohols
Compound |
Results of the Chromic Acid Test |
Results of the Potassium Permanganate Test |
Results of the Ferric Chloride Test |
|---|---|---|---|
|
Ethanol |
|||
|
Isopropyl alcohol |
|||
|
2-methyl-2-propanol |
|||
|
Cyclohexanol |
|||
|
Phenol |
|||
|
Unknown |
Analysis of Unknown Alcohol:
Unknown number OR Letter =
Solubility in water:
pH:
Results of chromic acid test:
Results of the potassium permanganate test:
Results of the ferric chloride test:
Based on the above results, identify the unknown alcohol as one of the five known:
Is your result conclusive? Explain.
POST-LAB QUESTIONS
1) For parts B and C, explain what happens when NaOH is added to insoluble alcohols and when HCl is added to insoluble amines. What type of reaction is occurring here?
2) Part A: Look at the boiling points of phenol and aniline. How do the boiling points relate to the results from part D?
Part B: Look up the boiling points for the following pairs of compounds:
Ethanol and ethanamine:
1-butanol and 1-butanamine:
Cyclohexanol and cyclohexanamine:
Part C: What do your answers for parts A and B of this question tell you about hydrogen bonding in alcohols and amines?
3) Draw structures for oxidation products of known alcohols that reacted with the chromic acid reagent in part F.
4) An unknown with molecular formula C4H10O showed results with the following chemical tests:
|
Chromic acid test |
Formation of orange solution |
|---|---|
|
Potassium permanganate test |
Formation of purple solution; after 10 min, two drops of 6 M HCl were added, and the purple solution.n remains |
|
Ferric chloride test |
Formation of yellow solution |
Using only this information, draw two possible structures for the unknown.
Please click here to access the Pre-Lab, Data Tables, and Post-Lab in Word or PDF format. Complete them and upload the lab report according to your instructor's instructions.


