5.E: Advanced Theories of Covalent Bonding (Exercises)
- Page ID
- 393239
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Molecular Structure and Polarity
Explain why the HOH molecule is bent, whereas the HBeH molecule is linear.
- Answer
-
The placement of the two sets of unpaired electrons in water forces the bonds to assume a tetrahedral arrangement, and the resulting HOH molecule is bent. The HBeH molecule (in which Be has only two electrons to bond with the two electrons from the hydrogens) must have the electron pairs as far from one another as possible and is therefore linear.
What feature of a Lewis structure can be used to tell if a molecule’s (or ion’s) electron-pair geometry and molecular structure will be identical?
- Answer
-
Add texts here. Do not delete this text first.
Explain the difference between electron-pair geometry and molecular structure.
- Answer
-
Space must be provided for each pair of electrons whether they are in a bond or are present as lone pairs. Electron-pair geometry considers the placement of all electrons. Molecular structure considers only the bonding-pair geometry.
Why is the H–N–H angle in NH3 smaller than the H–C–H bond angle in CH4? Why is the H–N–H angle in \(\ce{NH4+}\) identical to the H–C–H bond angle in CH4?
- Answer
-
Add texts here. Do not delete this text first.
Explain how a molecule that contains polar bonds can be nonpolar.
- Answer
-
As long as the polar bonds are compensated (for example. two identical atoms are found directly across the central atom from one another), the molecule can be nonpolar.
As a general rule, MXn molecules (where M represents a central atom and X represents terminal atoms; n = 2 – 5) are polar if there is one or more lone pairs of electrons on M. NH3 (M = N, X = H, n = 3) is an example. There are two molecular structures with lone pairs that are exceptions to this rule. What are they?
- Answer
-
Add texts here. Do not delete this text first.
Predict the electron pair geometry and the molecular structure of each of the following molecules or ions:
- SF6
- PCl5
- BeH2
- \(\ce{CH3+}\)
- Answer
-
- Both the electron geometry and the molecular structure are octahedral.
- Both the electron geometry and the molecular structure are trigonal bipyramid.
- Both the electron geometry and the molecular structure are linear.
- Both the electron geometry and the molecular structure are trigonal planar.
Identify the electron pair geometry and the molecular structure of each of the following molecules or ions:
- \(\ce{IF6+}\)
- CF4
- BF3
- \(\ce{SiF5-}\)
- BeCl2
- Answer
-
Add texts here. Do not delete this text first.
- What are the electron-pair geometry and the molecular structure of each of the following molecules or ions?
- ClF5
- \(\ce{ClO2-}\)
- \(\ce{TeCl4^2-}\)
- PCl3
- SeF4
- \(\ce{PH2-}\)
- Answer
-
- electron-pair geometry: octahedral, molecular structure: square pyramidal
- electron-pair geometry: tetrahedral, molecular structure: bent
- electron-pair geometry: octahedral, molecular structure: square planar
- electron-pair geometry: tetrahedral, molecular structure: trigonal pyramidal
- electron-pair geometry: trigonal bypyramidal, molecular structure: seesaw
- electron-pair geometry: tetrahedral, molecular structure: bent (109°)
Predict the electron pair geometry and the molecular structure of each of the following ions:
- H3O+
- \(\ce{PCl4-}\)
- \(\ce{SnCl3-}\)
- \(\ce{BrCl4-}\)
- ICl3
- XeF4
- SF2
- Answer
-
Add texts here. Do not delete this text first.
Identify the electron pair geometry and the molecular structure of each of the following molecules:
- ClNO (N is the central atom)
- CS2
- Cl2CO (C is the central atom)
- Cl2SO (S is the central atom)
- SO2F2 (S is the central atom)
- XeO2F2 (Xe is the central atom)
- \(\ce{ClOF2+}\) (Cl is the central atom)
- Answer
-
- electron-pair geometry: trigonal planar, molecular structure: bent (120°)
- electron-pair geometry: linear, molecular structure: linear
- electron-pair geometry: trigonal planar, molecular structure: trigonal planar
- electron-pair geometry: tetrahedral, molecular structure: trigonal pyramidal
- electron-pair geometry: tetrahedral, molecular structure: tetrahedral
- electron-pair geometry: trigonal bipyramidal, molecular structure: seesaw
- electron-pair geometry: tetrahedral, molecular structure: trigonal pyramidal
Predict the electron pair geometry and the molecular structure of each of the following:
- IOF5 (I is the central atom)
- POCl3 (P is the central atom)
- Cl2SeO (Se is the central atom)
- ClSO+ (S is the central atom)
- F2SO (S is the central atom)
- \(\ce{NO2-}\)
- \(\ce{SiO4^4-}\)
- Answer
-
Add texts here. Do not delete this text first.
- Which of the following molecules and ions contain polar bonds? Which of these molecules and ions have dipole moments?
- ClF5
- \(\ce{ClO2-}\)
- \(\ce{TeCl4^2-}\)
- PCl3
- SeF4
- \(\ce{PH2-}\)
- XeF2
- Answer
-
All of these molecules and ions contain polar bonds. Only ClF5, \(\ce{ClO2-}\), PCl3, SeF4, and \(\ce{PH2-}\) have dipole moments.
Which of the molecules and ions contain polar bonds? Which of these molecules and ions have dipole moments?
- H3O+
- \(\ce{PCl4-}\)
- \(\ce{SnCl3-}\)
- \(\ce{BrCl4-}\)
- ICl3
- XeF4
- SF2
- Answer
-
Add texts here. Do not delete this text first.
Which of the following molecules have dipole moments?
- CS2
- SeS2
- CCl2F2
- PCl3 (P is the central atom)
- ClNO (N is the central atom)
- Answer
-
SeS2, CCl2F2, PCl3, and ClNO all have dipole moments.
Identify the molecules with a dipole moment:
- SF4
- CF4
- Cl2CCBr2
- CH3Cl
- H2CO
- Answer
-
Add texts here. Do not delete this text first.
The molecule XF3 has a dipole moment. Is X boron or phosphorus?
- Answer
-
P
The molecule XCl2 has a dipole moment. Is X beryllium or sulfur?
- Answer
-
S
Is the Cl2BBCl2 molecule polar or nonpolar?
- Answer
-
nonpolar
There are three possible structures for PCl2F3 with phosphorus as the central atom. Draw them and discuss how measurements of dipole moments could help distinguish among them.
- Answer
-
Add texts here. Do not delete this text first.
Describe the molecular structure around the indicated atom or atoms:
- the sulfur atom in sulfuric acid, H2SO4 [(HO)2SO2]
- the chlorine atom in chloric acid, HClO3 [HOClO2]
- the oxygen atom in hydrogen peroxide, HOOH
- the nitrogen atom in nitric acid, HNO3 [HONO2]
- the oxygen atom in the OH group in nitric acid, HNO3 [HONO2]
- the central oxygen atom in the ozone molecule, O3
- each of the carbon atoms in propyne, CH3CCH
- the carbon atom in Freon, CCl2F2
- each of the carbon atoms in allene, H2CCCH2
- Answer
-
- tetrahedral
- trigonal pyramidal
- bent (109°)
- trigonal planar
- bent (109°)
- bent (109°)
- CH3CCH tetrahedral, CH3CCH linear
- tetrahedral
- H2CCCH2 linear, H2CCCH2 trigonal planar
Draw the Lewis structures and predict the shape of each compound or ion:
- CO2
- \(\ce{NO2-}\)
- SO3
- \(\ce{SO3^2-}\)
- Answer
-
Add texts here. Do not delete this text first.
A molecule with the formula AB2, in which A and B represent different atoms, could have one of three different shapes. Sketch and name the three different shapes that this molecule might have. Give an example of a molecule or ion for each shape.
- Answer
-

A molecule with the formula AB3, in which A and B represent different atoms, could have one of three different shapes. Sketch and name the three different shapes that this molecule might have. Give an example of a molecule or ion that has each shape.
- Answer
-
Add texts here. Do not delete this text first.
- Draw the Lewis electron dot structures for these molecules, including resonance structures where appropriate:
- \(\ce{CS3^2-}\)
- CS2
- CS
- Answer
-
Add texts here. Do not delete this text first.
- predict the molecular shapes for \(\ce{CS3^2-}\) and CS2 and explain how you arrived at your predictions
(a)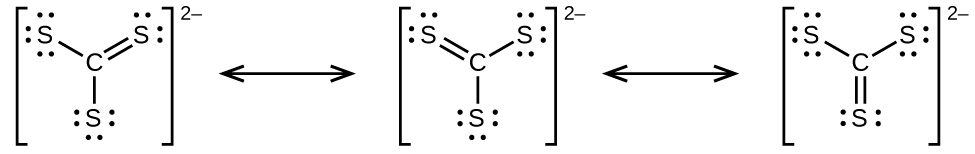
(b)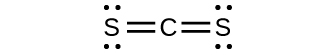
(c)
- Answer
-
\(\ce{CS3^2-}\) includes three regions of electron density (all are bonds with no lone pairs); the shape is trigonal planar;
CS2 has only two regions of electron density (all bonds with no lone pairs);
the shape is linear
What is the molecular structure of the stable form of FNO2? (N is the central atom.)
- Answer
-
Add texts here. Do not delete this text first.
A compound with a molar mass of about 42 g/mol contains 85.7% carbon and 14.3% hydrogen. What is its molecular structure?
- Answer
-
Add texts here. Do not delete this text first.
Use the simulation to perform the following exercises for a two-atom molecule:
- Adjust the electronegativity value so the bond dipole is pointing toward B. Then determine what the electronegativity values must be to switch the dipole so that it points toward A.
- With a partial positive charge on A, turn on the electric field and describe what happens.
- With a small partial negative charge on A, turn on the electric field and describe what happens.
- Reset all, and then with a large partial negative charge on A, turn on the electric field and describe what happens.
Use the simulation to perform the following exercises for a real molecule. You may need to rotate the molecules in three dimensions to see certain dipoles.
- Sketch the bond dipoles and molecular dipole (if any) for O3. Explain your observations.
- Look at the bond dipoles for NH3. Use these dipoles to predict whether N or H is more electronegative.
- Predict whether there should be a molecular dipole for NH3 and, if so, in which direction it will point. Check the molecular dipole box to test your hypothesis.
- Answer
-
The molecular dipole points away from the hydrogen atoms.
Use the Molecule Shape simulator to build a molecule. Starting with the central atom, click on the double bond to add one double bond. Then add one single bond and one lone pair. Rotate the molecule to observe the complete geometry. Name the electron group geometry and molecular structure and predict the bond angle.
- Answer
-
Click the check boxes at the bottom and right of the simulator to check your answers.
Use the Molecule Shape simulator to explore real molecules. On the Real Molecules tab, select H2O. Switch between the “real” and “model” modes. Explain the difference observed.
- Answer
-
The structures are very similar. In the model mode, each electron group occupies the same amount of space, so the bond angle is shown as 109.5°. In the “real” mode, the lone pairs are larger, causing the hydrogens to be compressed. This leads to the smaller angle of 104.5°.
Use the Molecule Shape simulator to explore real molecules. On the Real Molecules tab, select “model” mode and S2O. What is the model bond angle? Explain whether the “real” bond angle should be larger or smaller than the ideal model angle.
- Answer
-
Add texts here. Do not delete this text first.
Valence Bond Theory
Explain how σ and π bonds are similar and how they are different.
- Answer
-
Similarities: Both types of bonds result from overlap of atomic orbitals on adjacent atoms and contain a maximum of two electrons. Differences: σ bonds are stronger and result from end-to-end overlap and all single bonds are σ bonds; π bonds between the same two atoms are weaker because they result from side-by-side overlap, and multiple bonds contain one or more π bonds (in addition to a σ bond).
Draw a curve that describes the energy of a system with H and Cl atoms at varying distances. Then, find the minimum energy of this curve two ways.
- Use the bond energy found in Table 8.2.1 to calculate the energy for one single HCl bond (Hint: How many bonds are in a mole?)
- Use the enthalpy of reaction and the bond energies for \(H_2\) and \(Cl_2\) to solve for the energy of one mole of HCl bonds. \[H_{2(g)}+Cl_{2(g)} \rightleftharpoons 2HCl_{(g)} \;\;\; ΔH^∘_{rxn}=−184.7\; kJ/mol\]
- Answer
-
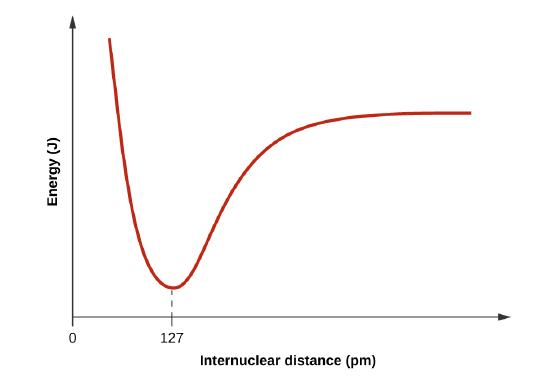
When H and Cl are separate (the x axis) the energy is at a particular value. As they approach, it decreases to a minimum at 127 pm (the bond distance), and then it increases sharply as you get closer.
- (a) H–Cl431 kJ/mol 427kJmol×mol6.022×1023bonds×1000 JkJ=7.09×10−19
- (b) We know Hess’s law related to bond energies: ΔH°=ƩΔH∘BDE(broken)−ƩΔH∘BDE(formed) We are given the enthalpy of reaction
\[−184.7 kJ/mol=(ΔH∘BDE(H–H)+ΔH∘BDE(Cl–Cl))−(2ΔH∘BDE(H–Cl))\]
\[H–H is 436 kJ/mol and Cl–Cl is 243\]
\[–184.7 kJ/mol = (436 + 243) – 2x = 679 – 2x\]
\[2x = 863.7 kJ/mol\]
\[x = 432\; kJ/mol\]
This is very close to the value from part (a).
Explain why bonds occur at specific average bond distances instead of the atoms approaching each other infinitely close.
- Answer
-
The specific average bond distance is the distance with the lowest energy. At distances less than the bond distance, the positive charges on the two nuclei repel each other, and the overall energy increases.
Use valence bond theory to explain the bonding in F2, HF, and ClBr. Sketch the overlap of the atomic orbitals involved in the bonds.
- Answer
-
The single bond present in each molecule results from overlap of the relevant orbitals: F 2p orbitals in F2, the H 1s and F 2p orbitals in HF, and the Cl 3p orbital and Br 4p orbital in ClBr.
Use valence bond theory to explain the bonding in O2. Sketch the overlap of the atomic orbitals involved in the bonds in O2.
- Answer
-
Bonding: One σ bond and one π bond. The s orbitals are filled and do not overlap. The p orbitals overlap along the axis to form a σ bond and side by side to form the π bond.
How many σ and π bonds are present in the molecule HCN?
- Answer
-
\(\ce{H–C≡N}\) has two σ (H–C and C–N) and two π (making the CN triple bond).

A friend tells you N2 has three π bonds due to overlap of the three p-orbitals on each N atom. Do you agree?
- Answer
-
No, two of the p orbitals (one on each N) will be oriented end-to-end and will form a σ bond.
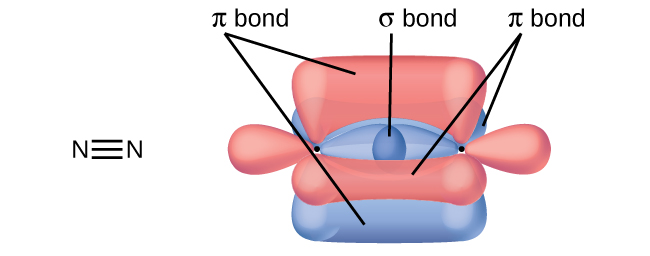
Draw the Lewis structures for CO2 and CO, and predict the number of σ and π bonds for each molecule.
- CO2
- CO
- Answer
-
(a) 2 σ 2 π;
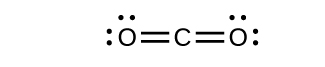
(b) 1 σ 2 π;
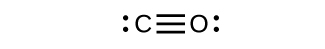
Hybrid Atomic Orbitals
Why is the concept of hybridization required in valence bond theory?
- Answer
-
Hybridization is introduced to explain the geometry of bonding orbitals in valance bond theory.Add texts here.
Give the shape that describes each hybrid orbital set:
(a) sp2
(b) sp3d
(c) sp
(d) sp3d2
- Answer
-
Add texts here. Do not delete this text first.
Explain why a carbon atom cannot form five bonds using sp3d hybrid orbitals.
- Answer
-
There are no d orbitals in the valence shell of carbon.
What is the hybridization of the central atom in each of the following?
(a) BeH2
(b) SF6
(c) \(\ce{PO4^3-}\)
(d) PCl5
- Answer
-
Add texts here. Do not delete this text first.
A molecule with the formula AB3 could have one of four different shapes. Give the shape and the hybridization of the central A atom for each.
- Answer
-
trigonal planar, sp2; trigonal pyramidal (one lone pair on A) sp3; T-shaped (two lone pairs on A sp3d, or (three lone pairs on A) sp3d2
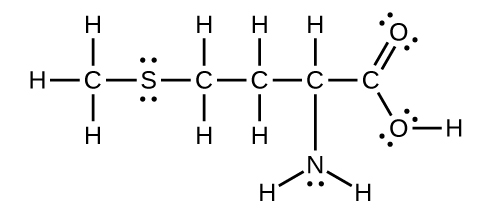
Methionine, CH3SCH2CH2CH(NH2)CO2H, is an amino acid found in proteins. Draw a Lewis structure of this compound. What is the hybridization type of each carbon, oxygen, the nitrogen, and the sulfur?
- Answer
-
Sulfuric acid is manufactured by a series of reactions represented by the following equations:
\(\ce{S8}(s)+\ce{8O2}(g)⟶\ce{8SO2}(g)\)
\(\ce{2SO2}(g)+\ce{O2}(g)⟶\ce{2SO3}(g)\)
\(\ce{SO3}(g)+\ce{H2O}(l)⟶\ce{H2SO4}(l)\)
Draw a Lewis structure, predict the molecular geometry by VSEPR, and determine the hybridization of sulfur for the following:
(a) circular S8 molecule
(b) SO2 molecule
(c) SO3 molecule
(d) H2SO4 molecule (the hydrogen atoms are bonded to oxygen atoms)
- Answer
-
(a) Each S has a bent (109°) geometry, sp3

(b) Bent (120°), sp2
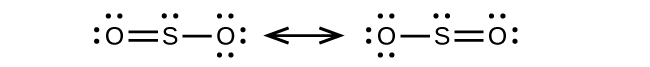
(c) Trigonal planar, sp2
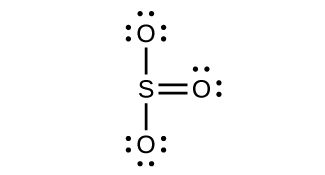
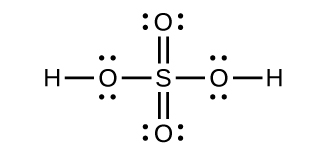
(d) Tetrahedral, sp3
Two important industrial chemicals, ethene, C2H4, and propene, C3H6, are produced by the steam (or thermal) cracking process:
\(\ce{2C3H8}(g)⟶\ce{C2H4}(g)+\ce{C3H6}(g)+\ce{CH4}(g)+\ce{H2}(g)\)
For each of the four carbon compounds, do the following:
(a) Draw a Lewis structure.
(b) Predict the geometry about the carbon atom.
(c) Determine the hybridization of each type of carbon atom.
- Answer
-
Add texts here. Do not delete this text first.
For many years after they were discovered, it was believed that the noble gases could not form compounds. Now we know that belief to be incorrect. A mixture of xenon and fluorine gases, confined in a quartz bulb and placed on a windowsill, is found to slowly produce a white solid. Analysis of the compound indicates that it contains 77.55% Xe and 22.45% F by mass.
(a) What is the formula of the compound?
(b) Write a Lewis structure for the compound.
(c) Predict the shape of the molecules of the compound.
(d) What hybridization is consistent with the shape you predicted?
Answer
-
(a) XeF2
(b)
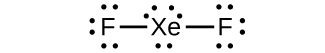
(c) linear
(d) sp3d
Consider nitrous acid, HNO2 (HONO).
(a) Write a Lewis structure.
(b) What are the electron pair and molecular geometries of the internal oxygen and nitrogen atoms in the HNO2 molecule?
(c) What is the hybridization on the internal oxygen and nitrogen atoms in HNO2?
- Answer
-
Add texts here. Do not delete this text first.
Strike-anywhere matches contain a layer of KClO3 and a layer of P4S3. The heat produced by the friction of striking the match causes these two compounds to react vigorously, which sets fire to the wooden stem of the match. KClO3 contains the \(\ce{ClO3-}\) ion. P4S3 is an unusual molecule with the skeletal structure.

(a) Write Lewis structures for P4S3 and the \(\ce{ClO3-}\) ion.
(b) Describe the geometry about the P atoms, the S atom, and the Cl atom in these species.
(c) Assign a hybridization to the P atoms, the S atom, and the Cl atom in these species.
(d) Determine the oxidation states and formal charge of the atoms in P4S3 and the \(\ce{ClO3-}\) ion.
- Answer
-
(a)

(b) P atoms, trigonal pyramidal; S atoms, bent, with two lone pairs; Cl atoms, trigonal pyramidal;
(c) Hybridization about P, S, and Cl is, in all cases, sp3;
(d) Oxidation states P +1, S \(−1\dfrac{1}{3}\), Cl +5, O –2. Formal charges: P 0; S 0; Cl +2: O –1
Identify the hybridization of each carbon atom in the following molecule. (The arrangement of atoms is given; you need to determine how many bonds connect each pair of atoms.)

- Answer
-
Add texts here. Do not delete this text first.
Write Lewis structures for NF3 and PF5. On the basis of hybrid orbitals, explain the fact that NF3, PF3, and PF5 are stable molecules, but NF5 does not exist.
- Answer
-

Phosphorus and nitrogen can form sp3 hybrids to form three bonds and hold one lone pair in PF3 and NF3, respectively. However, nitrogen has no valence d orbitals, so it cannot form a set of sp3d hybrid orbitals to bind five fluorine atoms in NF5. Phosphorus has d orbitals and can bind five fluorine atoms with sp3d hybrid orbitals in PF5.
In addition to NF3, two other fluoro derivatives of nitrogen are known: N2F4 and N2F2. What shapes do you predict for these two molecules? What is the hybridization for the nitrogen in each molecule?
- Answer
-
Add texts here. Do not delete this text first.
Multiple Bonds
The bond energy of a C–C single bond averages 347 kJ mol−1; that of a C≡C triple bond averages 839 kJ mol−1. Explain why the triple bond is not three times as strong as a single bond.
- Answer
-
Add texts here. Do not delete this text first.
A triple bond consists of one σ bond and two π bonds. A σ bond is stronger than a π bond due to greater overlap.
For the carbonate ion, \(\ce{CO3^2-}\), draw all of the resonance structures. Identify which orbitals overlap to create each bond.
- Answer
-
Add texts here. Do not delete this text first.
A useful solvent that will dissolve salts as well as organic compounds is the compound acetonitrile, H3CCN. It is present in paint strippers.
(a) Write the Lewis structure for acetonitrile, and indicate the direction of the dipole moment in the molecule.
(b) Identify the hybrid orbitals used by the carbon atoms in the molecule to form σ bonds.
(c) Describe the atomic orbitals that form the π bonds in the molecule. Note that it is not necessary to hybridize the nitrogen atom.
- Answer
-
(a)

(b) The terminal carbon atom uses sp3 hybrid orbitals, while the central carbon atom is sp hybridized.
(c) Each of the two π bonds is formed by overlap of a 2p orbital on carbon and a nitrogen 2p orbital.
For the molecule allene, \(\mathrm{H_2C=C=CH_2}\), give the hybridization of each carbon atom. Will the hydrogen atoms be in the same plane or perpendicular planes?
- Answer
-
Add texts here. Do not delete this text first.
Identify the hybridization of the central atom in each of the following molecules and ions that contain multiple bonds:
(a) ClNO (N is the central atom)
(b) CS2
(c) Cl2CO (C is the central atom)
(d) Cl2SO (S is the central atom)
(e) SO2F2 (S is the central atom)
(f) XeO2F2 (Xe is the central atom)
(g) \(\ce{ClOF2+}\) (Cl is the central atom)
- Answer
-
(a) sp2; (b) sp; (c) sp2; (d) sp3; (e) sp3; (f) sp3d; (g) sp3
Describe the molecular geometry and hybridization of the N, P, or S atoms in each of the following compounds.
(a) H3PO4, phosphoric acid, used in cola soft drinks
(b) NH4NO3, ammonium nitrate, a fertilizer and explosive
(c) S2Cl2, disulfur dichloride, used in vulcanizing rubber
(d) K4[O3POPO3], potassium pyrophosphate, an ingredient in some toothpastes
- Answer
-
Add texts here. Do not delete this text first.
For each of the following molecules, indicate the hybridization requested and whether or not the electrons will be delocalized:
(a) ozone (O3) central O hybridization
(b) carbon dioxide (CO2) central C hybridization
(c) nitrogen dioxide (NO2) central N hybridization
(d) phosphate ion (\(\ce{PO4^3-}\)) central P hybridization
- Answer
-
(a) sp2, delocalized; (b) sp, localized; (c) sp2, delocalized; (d) sp3, delocalized
For each of the following structures, determine the hybridization requested and whether the electrons will be delocalized:
(a) Hybridization of each carbon 
(b) Hybridization of sulfur 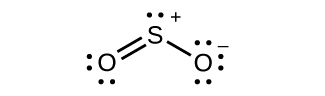
(c) All atoms 
- Answer
-
Add texts here. Do not delete this text first.
Draw the orbital diagram for carbon in CO2 showing how many carbon atom electrons are in each orbital.
- Answer
-
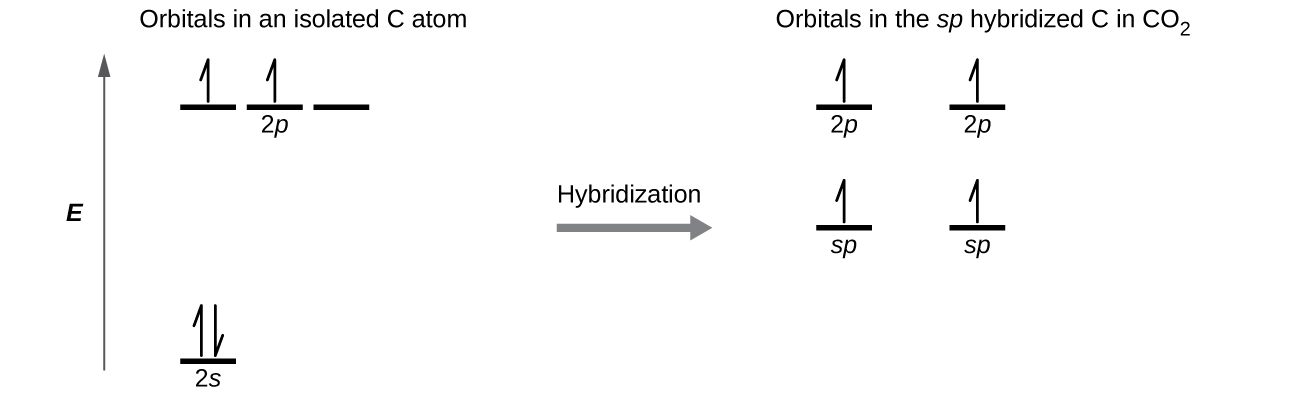 Each of the four electrons is in a separate orbital and overlaps with an electron on an oxygen atom.
Each of the four electrons is in a separate orbital and overlaps with an electron on an oxygen atom.
Molecular Orbital Theory
Sketch the distribution of electron density in the bonding and antibonding molecular orbitals formed from two s orbitals and from two p orbitals.
How are the following similar, and how do they differ?
(a) σ molecular orbitals and π molecular orbitals
(b) ψ for an atomic orbital and ψ for a molecular orbital
(c) bonding orbitals and antibonding orbitals
- Answer
-
(a) Similarities: Both are bonding orbitals that can contain a maximum of two electrons. Differences: σ orbitals are end-to-end combinations of atomic orbitals, whereas π orbitals are formed by side-by-side overlap of orbitals.
(b) Similarities: Both are quantum-mechanical constructs that represent the probability of finding the electron about the atom or the molecule. Differences: ψ for an atomic orbital describes the behavior of only one electron at a time based on the atom. For a molecule, ψ represents a mathematical combination of atomic orbitals.
(c) Similarities: Both are orbitals that can contain two electrons. Differences: Bonding orbitals result in holding two or more atoms together. Antibonding orbitals have the effect of destabilizing any bonding that has occurred.
If molecular orbitals are created by combining five atomic orbitals from atom A and five atomic orbitals from atom B combine, how many molecular orbitals will result?
- Answer
-
Add texts here. Do not delete this text first.
Can a molecule with an odd number of electrons ever be diamagnetic? Explain why or why not.
- Answer
-
An odd number of electrons can never be paired, regardless of the arrangement of the molecular orbitals. It will always be paramagnetic.
Can a molecule with an even number of electrons ever be paramagnetic? Explain why or why not.
- Answer
-
Add texts here. Do not delete this text first.
Why are bonding molecular orbitals lower in energy than the parent atomic orbitals?
- Answer
-
Bonding orbitals have electron density in close proximity to more than one nucleus. The interaction between the bonding positively charged nuclei and negatively charged electrons stabilizes the system.
Calculate the bond order for an ion with this configuration:
- Answer
-
Add texts here. Do not delete this text first.
Explain why an electron in the bonding molecular orbital in the H2 molecule has a lower energy than an electron in the 1s atomic orbital of either of the separated hydrogen atoms.
- Answer
-
The pairing of the two bonding electrons lowers the energy of the system relative to the energy of the nonbonded electrons.
Predict the valence electron molecular orbital configurations for the following, and state whether they will be stable or unstable ions.
(a) \(\ce{Na2^2+}\)
(b) \(\ce{Mg2^2+}\)
(c) \(\ce{Al2^2+}\)
(d) \(\ce{Si2^2+}\)
(e) \(\ce{P2^2+}\)
(f) \(\ce{S2^2+}\)
(g) \(\ce{F2^2+}\)
(h) \(\ce{Ar2^2+}\)
- Answer
-
Add texts here. Do not delete this text first.
Determine the bond order of each member of the following groups, and determine which member of each group is predicted by the molecular orbital model to have the strongest bond.
(a) H2, \(\ce{H2+}\), \(\ce{H2-}\)
(b) O2, \(\ce{O2^2+}\), \(\ce{O2^2-}\)
(c) Li2, \(\ce{Be2+}\), Be2
(d) F2, \(\ce{F2+}\), \(\ce{F2-}\)
- Answer
-
(a) H2 bond order = 1, \(\ce{H2+}\) bond order = 0.5, \(\ce{H2-}\) bond order = 0.5, strongest bond is H2;
(b) O2 bond order = 2, \(\ce{O2^2+}\) bond order = 3; \(\ce{O2^2-}\) bond order = 1, strongest bond is \(\ce{O2^2+}\);
(c) Li2 bond order = 1, \(\ce{Be2+}\) bond order = 0.5, Be2 bond order = 0, strongest bond is \(\ce{Li2}\);
(d) F2 bond order = 1, \(\ce{F2+}\) bond order = 1.5, \(\ce{F2-}\) bond order = 0.5, strongest bond is \(\ce{F2+}\);
For the first ionization energy for an N2 molecule, what molecular orbital is the electron removed from?
- Answer
-
Add texts here. Do not delete this text first.
Compare the atomic and molecular orbital diagrams to identify the member of each of the following pairs that has the highest first ionization energy (the most tightly bound electron) in the gas phase:
(a) H and H2
(b) N and N2
(c) O and O2
(d) C and C2
(e) B and B2
Answer
-
(a) H2; (b) N2; (c) O; (d) C2; (e) B2
Which of the period 2 homonuclear diatomic molecules are predicted to be paramagnetic?
- Answer
-
Add texts here. Do not delete this text first.
A friend tells you that the 2s orbital for fluorine starts off at a much lower energy than the 2s orbital for lithium, so the resulting σ2s molecular orbital in F2 is more stable than in Li2. Do you agree?
- Answer
-
Yes, fluorine is a smaller atom than Li, so atoms in the 2s orbital are closer to the nucleus and more stable.
True or false: Boron contains 2s22p1 valence electrons, so only one p orbital is needed to form molecular orbitals.
- Answer
-
Add texts here. Do not delete this text first.
What charge would be needed on F2 to generate an ion with a bond order of 2?
- Answer
-
2+
Predict whether the MO diagram for S2 would show s-p mixing or not.
- Answer
-
Add texts here. Do not delete this text first.
Explain why \(\ce{N2^2+}\) is diamagnetic, while \(\ce{O2^4+}\), which has the same number of valence electrons, is paramagnetic.
- Answer
-
N2 has s-p mixing, so the π orbitals are the last filled in \(\ce{N2^2+}\). O2 does not have s-p mixing, so the σp orbital fills before the π orbitals.
Using the MO diagrams, predict the bond order for the stronger bond in each pair:
(a) F2 or \(\ce{F2+}\)
(b) O2 or \(\ce{O2^2+}\)
Answer
-
Add texts here. Do not delete this text first.


