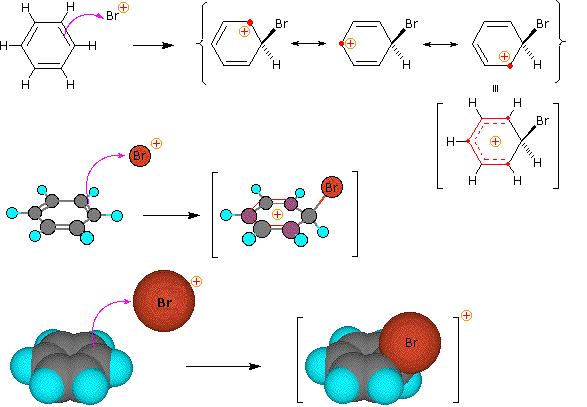14.2: Examples of electrophilic aromatic substitution
- Page ID
- 225861
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)A Detailed discussion of the mechanism for electrophilic substitution reactions of benzene
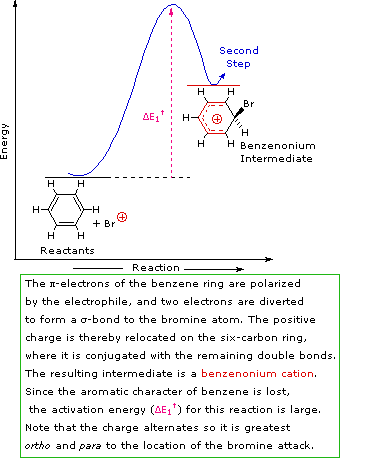
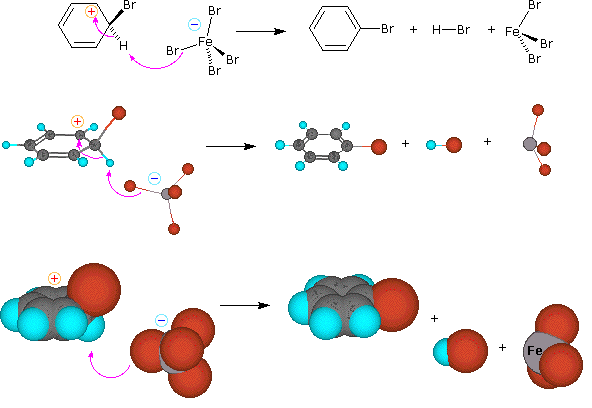
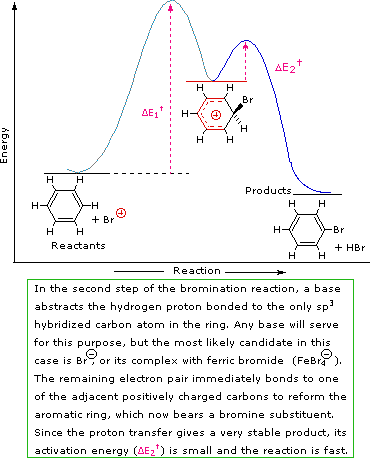
A two-step mechanism has been proposed for these electrophilic substitution reactions. In the first, slow or rate-determining, step the electrophile forms a sigma-bond to the benzene ring, generating a positively charged benzenonium intermediate. In the second, fast step, a proton is removed from this intermediate, yielding a substituted benzene ring. The following four-part illustration shows this mechanism for the bromination reaction. Also, an animated diagram may be viewed.

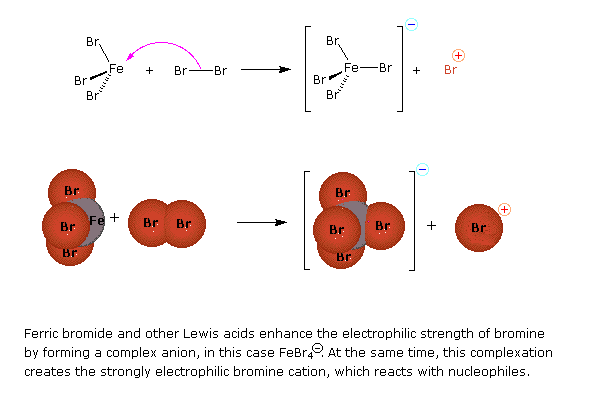
Preliminary step: Formation of the strongly electrophilic bromine cation
Step 1: The electrophile forms a sigma-bond to the benzene ring, generating a positively charged Wheland intermediate
Step 2: A proton is removed from this intermediate via an electrophile elimination reaction, yielding a substituted benzene ring
This mechanism for electrophilic aromatic substitution should be considered in context with other mechanisms involving carbocation intermediates. These include SN1 and E1 reactions of alkyl halides, and Brønsted acid addition reactions of alkenes.
To summarize, when carbocation intermediates are formed one can expect them to react further by one or more of the following modes:
1. The cation may bond to a nucleophile to give a substitution or addition product (coordination).
2. The cation may transfer a proton to a base, giving a double bond product (electrophile elimination).
3. The cation may rearrange to a more stable carbocation, and then react by mode #1 or #2.
SN1 and E1 reactions are respective examples of the first two modes of reaction. The second step of alkene addition reactions proceeds by the first mode, and any of these three reactions may exhibit molecular rearrangement if an initial unstable carbocation is formed. The carbocation intermediate in electrophilic aromatic substitution (the Wheland intermediate) is stabilized by charge delocalization (resonance) so it is not subject to rearrangement. In principle it could react by either mode 1 or 2, but the energetic advantage of reforming an aromatic ring leads to exclusive reaction by mode 2 (i.e., proton loss).
Synthesis of benzene derivatives via electrophilic aromatic substitution
| Reaction | Reagent | Catalyst | Product | E+ or E |
|---|---|---|---|---|
| Halogenation | X2 (X=Cl, Br) X2 (X = I) | FeX3 HNO3 | ArCl, ArBr ArI | X+ H2O−I+ |
| Nitration | HNO3 | H2SO4 | ArNO2 | +NO2 |
| Sulfonation | H2SO4 or H2S2O7 | None | ArSO3H | SO3 |
| Friedel-Crafts alkylation | RX, ArCH2X | AlCl3 | Ar-R, Ar-CH2Ar | R+ |
| ROH | HF, H2SO4, or BF3 | Ar-R | R+ | |
| RCH=CH2 | H3PO4 or HF | Ar-CHRCH3 | R+ | |
| Friedel-Crafts acylation | RCOCl | AlCl3 | Ar-COR | RC+=O |
Electrophilic aromatic substitution reactions – Halogenation
Study Note
The general mechanism is the key to understanding electrophilic aromatic substitution. You will see similar equations written for nitration, sulphonation, acylation, etc., but the general mechanism is always the same – the major difference being the identity of the electrophile in each case. All involve an electrophilic addition step which is quickly followed by an electrophile elimination step.
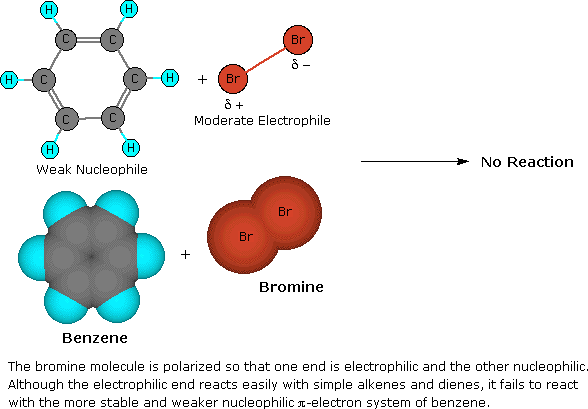
Halogenation is an example of electrophillic aromatic substitution. In electrophilic aromatic substitutions, a benzene is attacked by an electrophile which results in substition of hydrogens. However, halogens are not electrophillic enough to break the aromaticity of benzenes, which require a catalyst (such as FeCl3) to activate. See above for a detailed examination of the mechanism for bromination of benzene.
Exercises
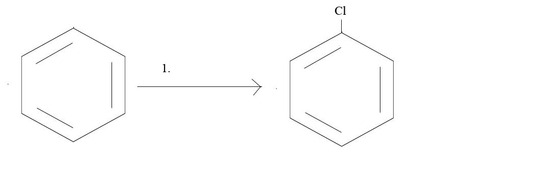
1. What reagents would you need to get the given product?
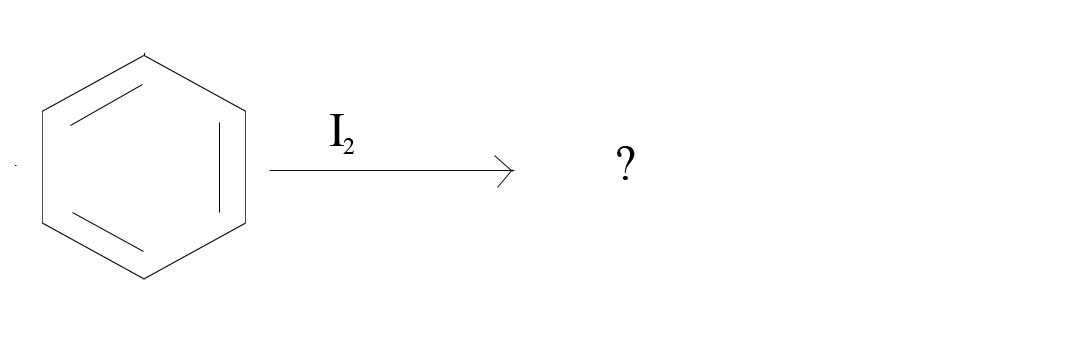
2. What product would result from the given reagents?
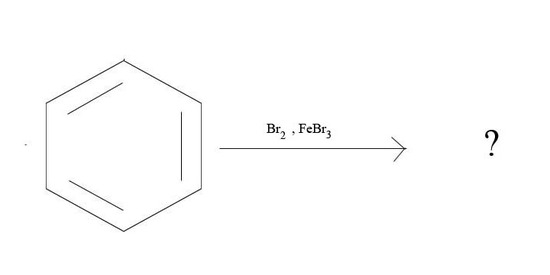
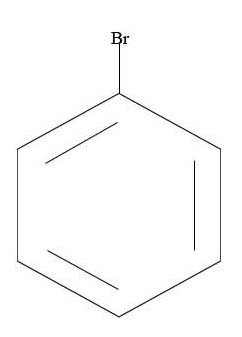
3. What is the major product given the reagents below?
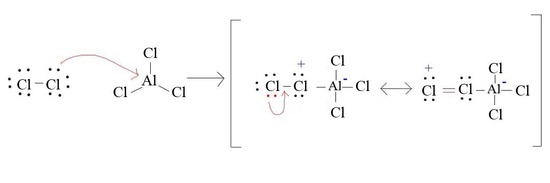
4. Draw the formation of Cl+ from AlCl3 and Cl2
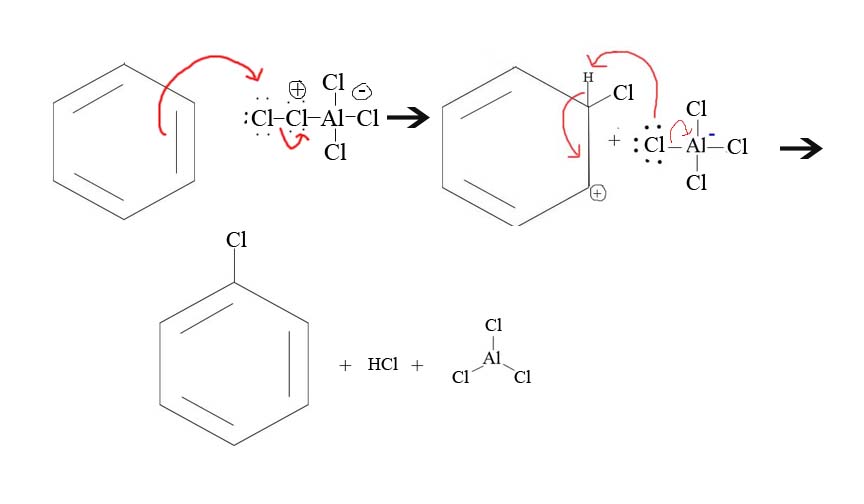
5. Draw the mechanism of the reaction between Cl+ and a benzene.
Solutions
[reveal-answer q=”789315″]Show Answer[/reveal-answer]
[hidden-answer a=”789315″]
1. Cl2 and AlCl3 or Cl2 and FeCl3
2. No Reaction
3.
4.
5.
 [/hidden-answer]
[/hidden-answer]
Nitration of Benzene
The source of the nitronium ion is through the protonation of nitric acid by sulfuric acid, which causes the loss of a water molecule and formation of a nitronium ion.
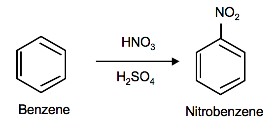
Sulfuric acid activation of nitric acid
The first step in the nitration of benzene is to activate HNO3 with sulfuric acid to produce a stronger electrophile, the nitronium ion.
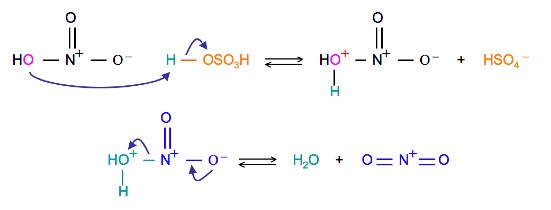
Because the nitronium ion is a good electrophile, it is attacked by benzene to produce nitrobenzene.
Mechanism
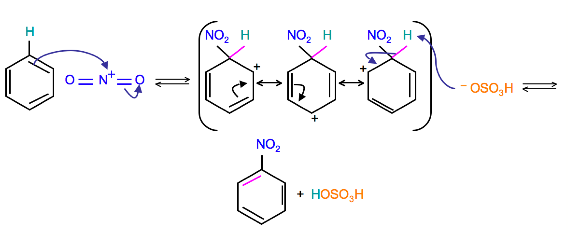
(Resonance forms of the intermediate can be seen in the generalized electrophilic aromatic substitution)
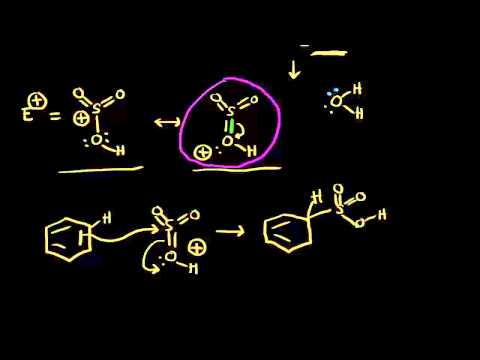
Sulfonation of benzene
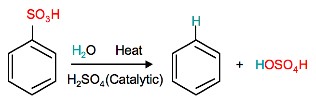
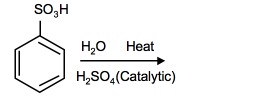
Sulfonation is a reversible reaction that produces benzenesulfonic acid by adding sulfur trioxide and fuming sulfuric acid. The reaction is reversed by adding hot aqueous acid to benzenesulfonic acid to produce benzene.
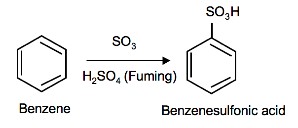
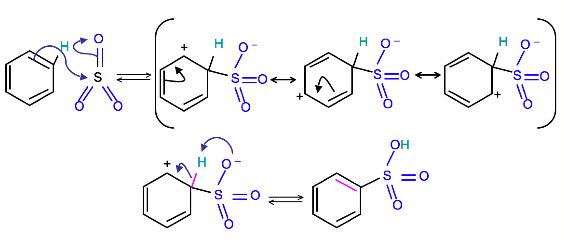
Mechanism
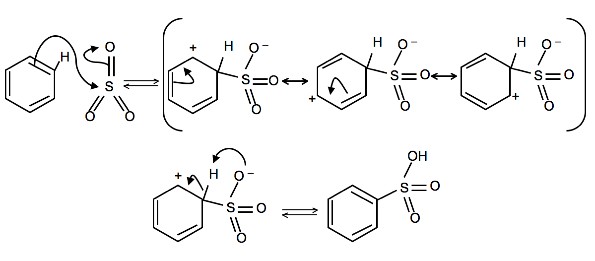
To produce benzenesulfonic acid from benzene, fuming sulfuric acid and sulfur trioxide are added. Fuming sulfuric acid, also refered to as oleum, is a concentrated solution of dissolved sulfur trioxide in sulfuric acid. The sulfur in sulfur trioxide is electrophilic because the oxygens pull electrons away from it because oxygen is very electronegative. The benzene attacks the sulfur (and subsequent proton transfers occur) to produce benzenesulfonic acid.
Reverse sulfonation
Sulfonation of benzene is a reversible reaction. Sulfur trioxide readily reacts with water to produce sulfuric acid and heat. Therefore, by adding heat to benzenesulfonic acid in diluted aqueous sulfuric acid the reaction is reversed.
Further applications of nitration and sulfonation
Nitration is used to add nitrogen to a benzene ring, which can be used further in substitution reactions. The nitro group acts as a ring deactivator. Having nitrogen present in a ring is very useful because it can be used as a directing group as well as a masked amino group. The products of aromatic nitrations are very important intermediates in industrial chemistry.
Because sulfonation is a reversible reaction, it can also be used in further substitution reactions in the form of a directing blocking group because it can be easily removed. The sulfonic group blocks the carbon from being attacked by other substituents and after the reaction is completed it can be removed by reverse sulfonation. Benzenesulfonic acids are also used in the synthesis of detergents, dyes, and sulfa drugs. Benzenesulfonyl chloride is a precursor to sulfonamides, which are used in chemotherapy.
Outside Links
Aromatic Sulfonation


- Interactive 3D Reaction: http://www.chemtube3d.com/Electrophi…20benzene.html


Aromatic Nitration
- Wikipedia: http://en.wikipedia.org/wiki/Nitration


- Interactive 3D Reaction: http://www.chemtube3d.com/Electrophi…20benzene.html


Exercises
1. What is/are the required reagent(s)for the following reaction:

2. What is the product of the following reaction:
3. Why is it important that the nitration of benzene by nitric acid occurs in sulfuric acid?
4. Write a detailed mechanism for the sulfonation of benzene, including all resonance forms.
5. Draw an energy diagram for the nitration of benzene. Draw the intermediates, starting materials, and products. Label the transition states.
Solutions
[reveal-answer q=”381436″]Show Answer[/reveal-answer]
[hidden-answer a=”381436″]
1. SO3 and H2SO4 (fuming)
2.
3. Sulfuric acid is needed in order for a good electrophile to form. Sulfuric acid protonates nitric acid to form the nitronium ion (water molecule is lost). The nitronium ion is a very good electrophile and is open to attack by benzene. Without sulfuric acid the reaction would not occur.
4.
5.
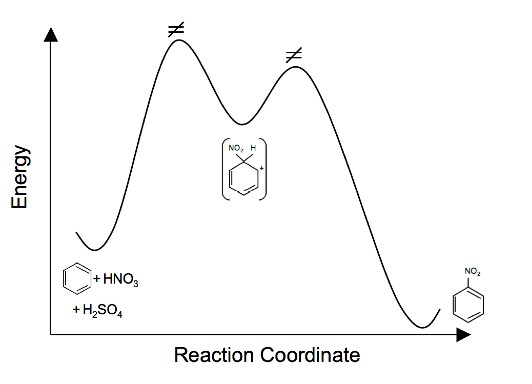 [/hidden-answer]
[/hidden-answer]
Reduction of Nitro groups
Electrophilic nitration introduces a deactivating, meta-directing substituents on an aromatic ring. The attached nitrogen is in a high oxidation state, and reduction converts the nitro into an electron donating amino group. Reduction is easily achieved either by catalytic hydrogenation (H2 + catalyst), or with reducing metals in acid. Examples of this reduction are shown here.
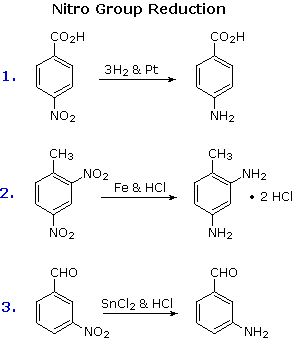
Several alternative methods for reducing nitro groups to amines are known. These include zinc or tin in dilute mineral acid, and sodium sulfide in aqueous ammonia solution. The procedures described above are sufficient for most cases.
References
- Laali, Kenneth K., and Volkar J. Gettwert. “Electrophilic Nitration of Aromatics in Ionic Liquid Solvents.” The Journal of Organic Chemistry 66 (Dec. 2000): 35-40. American Chemical Society.
- Malhotra, Ripudaman, Subhash C. Narang, and George A. Olah. Nitration: Methods and Mechanisms. New York: VCH Publishers, Inc., 1989.
- Sauls, Thomas W., Walter H. Rueggeberg, and Samuel L. Norwood. “On the Mechanism of Sulfonation of the Aromatic Nucleus and Sulfone Formation.” The Journal of Organic Chemistry 66 (1955): 455-465. American Chemical Society.
- Vollhardt, Peter. Organic Chemistry : Structure and Function. 5th ed. Boston: W. H. Freeman & Company, 2007.
Contributors
- Prof. Steven Farmer (Sonoma State University)
- William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
Contributors
- Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
- Prof. Steven Farmer (Sonoma State University)
- Catherine Nguyen
- William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
Contributors
- Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
- Prof. Steven Farmer (Sonoma State University)
- William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
- Mario Morataya (UCD)
- Gamini Gunawardena from the OChemPal site (Utah Valley University)
- 18.2: The General Mechanism. Authored by: Prof. Steven Farmer, William Reusch, Professor Emeritus . Provided by: Sonoma State University, Michigan State U. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Map%3A_Organic_Chemistry_(Smith)/Chapter_18%3A_Electrophilic_Aromatic_Substitution/18.2%3A_The_General_Mechanism. Project: Chemistry LibreTexts . License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- Synthesis of Benzene Derivatives: Electrophilic Aromatic Substitution. Authored by: Steve Maxwell. Provided by: UCD. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Arenes/Synthesis_of_Arenes/Electrophilic_Aromatic_Substitution. Project: Chemistry LibreTexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- 16.1: Electrophilic Aromatic Substitution Reactions - Bromination. Authored by: Dr. Dietmar Kennepohl FCIC; Prof. Steven Farmer; Catherine Nguyen; Wiliam Reusch, Professor Emeritus. Provided by: Athabasca University; Sonoma State University; Michigan State U. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Map%3A_Organic_Chemistry_(McMurry)/Chapter_16%3A_Chemistry_of_Benzene_-_Electrophilic_Aromatic_Substitution/16.01_Electrophilic_Aromatic_Substitution_Reactions%3A_Bromination. Project: Chemistry LibreTexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- 18.4: Nitration and Sulfonation. Authored by: Anonymous. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Map%3A_Organic_Chemistry_(Smith)/Chapter_18%3A_Electrophilic_Aromatic_Substitution/18.4%3A_Nitration_and_Sulfonation. Project: Chemistry LibreTexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- 16.10 Reduction of Aromatic Compounds. Authored by: Dr. Dietmar Kennepohl FCIC; Prof. Steven Farmer; William Reusch, Professor Emeritus; Mario Morataya; Gamini Gunawardena . Provided by: Athabasca University; Sonoma State University; Michigan State U; UCD; Utah Valley University. Located at: https://chem.libretexts.org/LibreTexts/Athabasca_University/Chemistry_350%3A_Organic_Chemistry_I/Chapter_16%3A_Chemistry_of_Benzene%3A_Electrophilic_Aromatic_Substitution/16.10_Reduction_of_Aromatic_Compounds. Project: Chemistry LibreTexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike