Synthesis of Benzene Derivatives: Electrophilic Aromatic Substitution
- Page ID
- 939
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)This section is on the general mechanism of how an electrophilic atom becomes a part of a benzene ring through the substitution of a hydrogen. Common reactions that proceed by electrophilic aromatic substitution include the nitration and sulfonation of benzene, hydration of benzene, friedel-crafts acylation and friedel-crafts alkylation.
Reactivity of benzene
Benzene is an aromatic compound that is greatly stabilized by its resonance forms. Stable compounds are much harder to react with, therefore a strong electrophile will be needed to attack the \(pi\) electrons in the benzene ring. Electrophiles used in alkene reactions will typically not be strong enough on their own, therefore a Lewis acid catalyst is required to help generate the electrophile. Although the general mechanism listed here starts with a non-substituted benzene ring, it should make sense that this same reaction could still occur even if there was already a constituent present on the ring, creating polysubstituted benzene rings.
| Reaction | Reagent | Catalyst | Product | E+ or E |
|---|---|---|---|---|
| Halogenation | X2 (X=Cl, Br) | FeX3 | ArCl, ArBr | X+ |
| Nitration | HNO3 | H2SO4 | ArNO2 | +NO2 |
| Sulfonation | H2SO4 or H2S2O7 | None | ArSO3H | SO3 |
| Friedel-Crafts alkylation | RX, ArCH2X | AlCl3 | Ar-R, Ar-CH2Ar | R+ |
| ROH | HF, H2SO4, or BF3 | Ar-R | R+ | |
| RCH=CH2 | H3PO4 or HF | Ar-CHRCH3 | R+ | |
| Friedel-Crafts acylation | RCOCl | AlCl3 | Ar-COR | RC+=O |
Potential Energy Diagram of Reaction
Below in a potential energy diagram showing the reaction course of benzene with an electrophile. Notice that the INTERMEDIATE IS NOT AROMATIC. Aromatic cyclic compounds are much more stable than cyclic alkenes, which is why the reaction will continue after the substitution until the aromaticity of the ring as been regained.
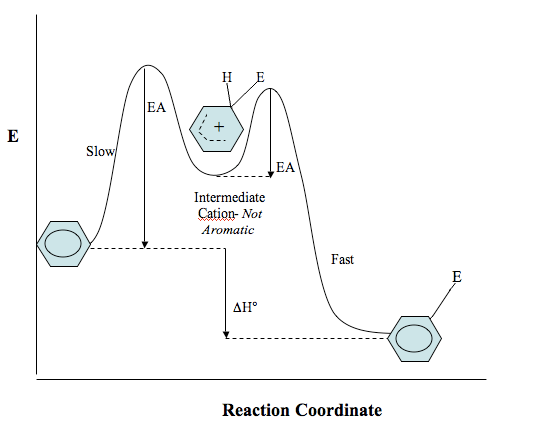
Step 1 has a high activation energy barrier because a non-aromatic intermediate is formed. Aromatic molecules are lower in energy than their non-aromatic counterparts, so this step is endothermic (product has higher energy than reactant). This step is slow and not thermodynamically favored because the product has higher energy than the reactant. From this intermediate there is a smaller energy of activation- this is because the reaction wants to proceed forward and regain the aromaticity lost in the first step, so this occurs quickly. The aromatic product has lower energy, so this step is exothermic. If you still have trouble interpreting this diagram, check out this site on reaction kinetics to review how to read potential energy diagrams.
References
- Klein, David R. Organic Chemistry II as a Second Language. Hoboken, NJ: John Wiley & Sons, 2006
- Parsons, A.F. Keynotes in Organic Chemistry. Oxford; Malden, MA: Blackwell Science, 2003
- Taylor, R. Electrophilic Aromatic Substitution. Chichester, West Sussex, England; New York: J. Wiley, 1990
Problems
- Label the hybridization on all the carbons in a) reacting benzene ring, b) intermediate (including resonance forms), and c) product (monosubstituted benzene ring)
- Is the energy of activation higher in the first step or second step of the mechanism? Explain your reasoning.
- If you wanted to halogenate benzene, what sort of reagent and catalyst (if needed) would you use?
- Which hydrogren is used in order to regain aromaticity after the electrophile has added to the ring?
- Critical Thinking Question: Mentioned above was the fact that electrophilic aromatic substitution can and does happen when there are substituents already present on the ring. Already present substituents will determine where something adds onto the ring in relation to itself (ortho, meta, or para position). What sort of factors do you think influence where addition will occur?
Answers
- a) All are sp2 b) Five carbons are sp2, the carbon with the attached E is sp3 hybridized c) All are sp2
- The activation energy is higher for the first step- aromatic compounds are lower in energy ("happier") than their non-aromatic forms. Therefore it takes more energy to change a compound from being aromatic to non-aromatic, than it does in the reverse direction.
- \(X_2 (X=Cl, Br) + FeX_3\)
- The hydrogen used is the one attached to the same carbon that E added to
- Critical Thinking: If you came up with a) Inductive Effects (correlates to electronegativity) b) Resonance effects c) Electrostatics d) Steric Hindrance then good job! All of these influence where a substituent will add to the ring. Go to Activating and Deactivating Benzene Rings to find out more about how position is determined.
Contributors
- Stevie Maxwell (UCD)

