AR4. Activation and Deactivation
- Page ID
- 4278
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Because the benzene acts as a nucleophile in electrophilic aromatic substitution, substituents that make the benzene more electron-rich can accelerate the reaction. Substituents that make the benzene moor electron-poor can retard the reaction. In the mid-twentieth century, physical organic chemists including Christopher Ingold conducted a number of kinetic studies on electrophilic aromatic substitution reactions. In table 1, you can see that some substituents confer a rate of reaction that is much higher than that of benzene (R = H). Phenol, C6H5OH, undergoes nitration a thousand times faster than benzene does. Nitrobenzene, C6H5NO2, undergoes the reaction millions of times more slowly.
| R in C6H5R | Relative rate |
| OH | 1,000 |
| CH3 | 25 |
| H | 1 |
| CH2Cl | 0.71 |
| I | 0.18 |
| F | 0.15 |
| Cl | 0.033 |
| Br | 0.030 |
| CO2Et | 0.0037 |
| NO2 | 6 x 10-8 |
| NMe3+ | 1.2 x 10-8 |
These observations are consistent with the role of the aromatic as a nucleophile in this reaction. Substituents that draw electron density away from the aromatic ring slow the reaction down. These groups are called deactivating groups in this reaction. Substituents that readily donate electron desnity to the ring, or that effectively stabilize the cationic intermediate, promote the reaction. These groups are called activating groups in this reaction.
The roles of these groups are related to their electronic interactions with the electrons in the ring. Some groups might be π-donors, providing additional electron density to the benzene ring via conjugation.
Other groups may be π-acceptors, drawing electron density away from the ring via conjugation.
Still others may be σ-acceptors, drawing electron density away from the ring via a simple inductive effect which arises from the electronegativity of a substituent.
In some cases, there may be multiple effects, and the overall influence of the substituents is determined by the balance of the effects. One effect may be stronger in one case than the other, so it wins out in one case and loses in another.
Problem AR4.1.
Explain why a fluorine atom would slow down an electrophilic substitution on an adjacent benzene ring.
Problem AR4.2.
Show, with structures, how the OH group in phenol makes the benzene ring more nucleophilic.
Problem AR4.3.
Show, with structures, how the CO2Et group makes the benzene ring less nucleophilic.
Problem AR4.4.
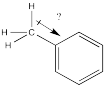
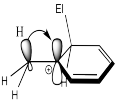
Show, with structures, how a methyl group stabilizes the cationic intermediate during a nitration reaction.
In general, deactivating groups fall into two classes. Π-acceptors, such as carbonyls, if placed directly adjacent to the aromatic ring, slow down the reaction. Highly electronegative atoms, typically halogens, attached directly to the aromatic ring also slow down the reaction.
- deactivating groups make electrophilic aromatic substitutionslower than in benzene
- π-acceptors are deactivating groups
- halogens are deactivating groups
Activating groups also fall into two categories. Π-donors, typically oxygen or nitrogen atoms, accelerate the reaction. This observation is true even though these atoms are also highly electronegative. Alkyl groups attached to the aromatic ring also accelerate the reaction.
- activating groups make electrophilic aromatic substitution faster than in benzene
- oxygen and nitrogen π-donors are activating groups
- alkyls are activating groups
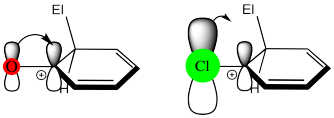
Note that halogens are also π-donors, but they are less effective in this regard than nitrogen or oxygen. That's because nitrogen and oxygen are similar in size to carbon, and they form effective π-overlap with the adjacent carbon on the benzene ring. In the halogens, electronegativity wins by default, because their π-donating effects are not good enough to make them activators.
Conversely, nitrogen and oxygen are both very electronegative, but their exceptional π-donating ability makes them activators rather than deactivators. Thus, in many cases, there is a subtle balance between activating and directing effects. In some cases, the activating effect is more pronounced, and that is what is observed. In other cases, it is the deactivating effect that wins out.
Alkyl groups behave almost as sigma donors, although that may be a misleading way to think about them.
Instead, their mild activating effect arises from hyperconjugation, in which a pair of C-H bonding electrons can weakly interact with a cationic site, providing a little extra stability to the cation.
Problem AR4.5.
One of the groups in the table, CH2Cl, does not quite fit the general rules. It is very slightly deactivating. Explain why this group acts in this way.
Problem AR4.6.
Predict whether each of the following groups would be activating or deactivating towards electrophilic aromatic substitution.
a) NH2 b) CN c) OCH3 d) SMe2+ e) C(O)CH3
Problem AR4.7.
Explain the trend in activating effects among the different halogens.