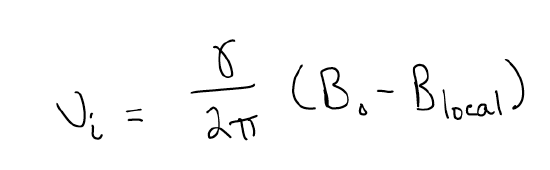5.2: Determining the Number of Signals in NMR Spectroscopy
- Page ID
- 319876
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Let’s take a look at how the structure of a molecule affects the number and type of peaks we see in the NMR spectrum. For simplicity, let’s focus only on 13C nuclei. The first thing we need to worry about is something known as magnetic equivalency. The total number of signals (or resonances) for a particular nucleus may not exceed the total number of those nuclei in the molecule, but it may be less. This happens when there is symmetry in the molecule, for example when there are mirror planes or rotational axes. Let’s look at a few examples and determine how many 13C signals would exist in an NMR spectrum. Benzene has the structure shown below. Since it has six carbon atoms, it’s NMR spectrum could have a total of six signals maximum. However, there are lines of symmetry in this molecule – six of them to be exact – which causes each of the carbon atoms to become magnetically equivalent. Therefore, we only see one signal in the NMR spectrum for benzene. All of the 13C nuclei in benzene are magnetically equivalent and share the same frequency.
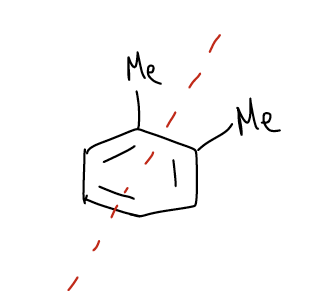
The next example is 1,2-dimethylbenzene. There are eight carbon atoms total, so the maximum number of signals is eight. However, there is a mirror plane through the center of the molecule. This reduces the number of signals in half because nuclei that are on either side of the mirror plane are magnetically equivalent. There are four signals total.
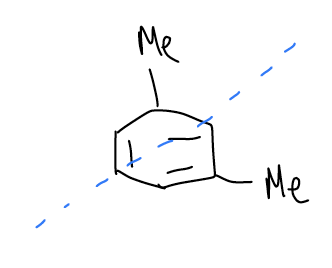
In 1,3-dimethylbenzene, there are still eight signals maximum, but this time there is a mirror plane that bisects the molecule in which two of the carbon atoms sit directly on the mirror plane. The total number of signals that we see is five because the nuclei that are on the mirror plane are unique.
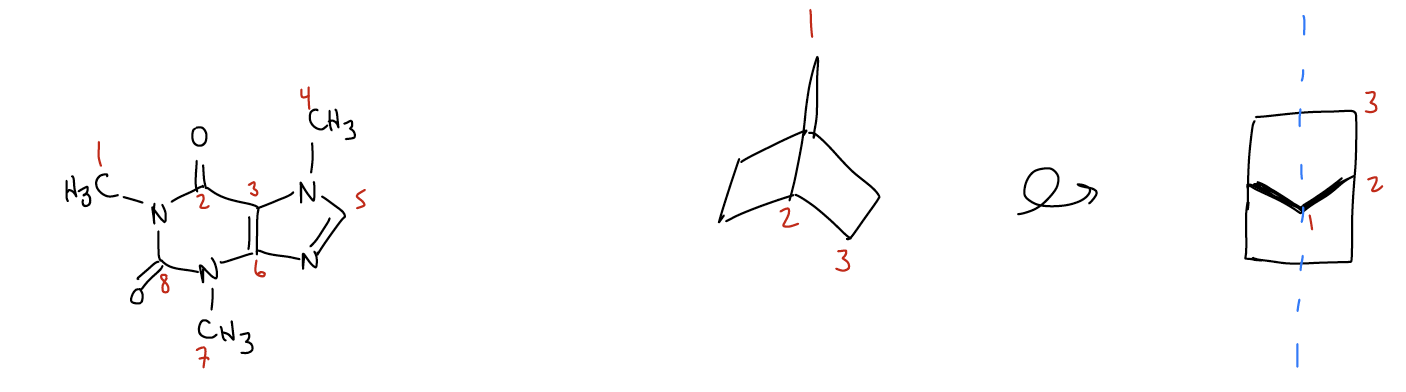
In certain cases, there is no element of symmetry. The molecule caffeine, for example, has eight carbons. There are no planes or axes of symmetry in caffeine, so we observe eight signals total. Finally, just because a molecule looks complex, such as bicyclo[2.2.1]heptane, does not mean that the 13C NMR spectrum is overly complex. There are a total of seven carbon atoms in this molecule, but a plane of symmetry exists in the molecule directly through the center. Thus, we see only three carbon resonances in the NMR spectrum. This is much easier to see if you were to build a model and rotate it so that you are looking at the molecule from the front.
The same symmetry rules apply for 1H nuclei. Go back and see if you can identify how many 1H signals you would find in the 1H NMR spectrum for each of the molecules above.
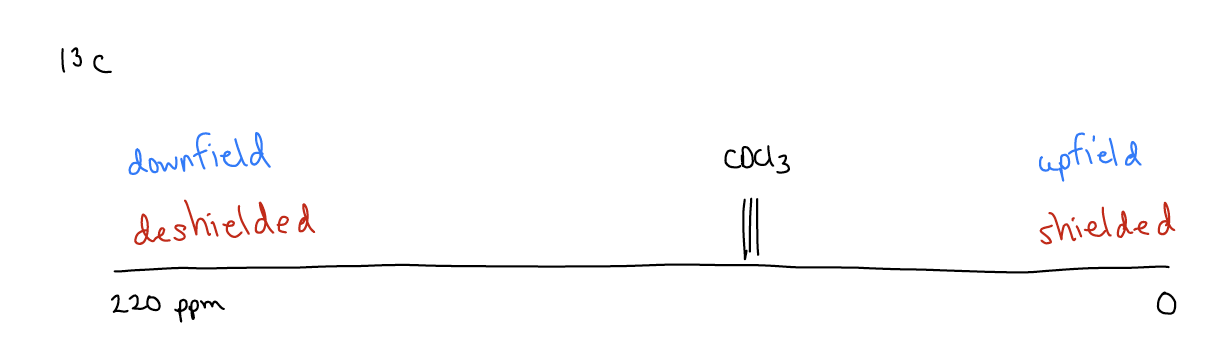
Another factor that affects any NMR spectrum is the chemical shift, which tells us about the electron density around a particular nucleus. This allows us to deduce the molecules’ connectivity. The typical 13C spectrum has a spectral width of 0-220 ppm. We normally dissolve our sample in deuterochloroform (CDCl3), which will give its own signal at 77.0 ppm. The right hand side of the spectrum (or lower ppm) is called upfield, and nuclei in this region are considered shielded, while the left hand side of the spectrum (higher ppm) is called downfield. We say that nuclei in this region are deshielded.
The Larmour equation will take into account small changes in the local environment, which will change the resonant frequency ever so slightly. How does this happen? Well, shielding will occur because the local environment will reduce the full effect of the applied magnetic field. This happens when more electron density surrounds a particular nucleus. Shielding will shift nuclei upfield. Deshielding, on the other hand, will shift nuclei downfield, because now the nuclei are experiencing the full effect of the magnetic field, and perhaps even more. Nuclei that do not have a lot of electron density around them will thus be shifted downfield.