5.1: The Physics of Nuclear Magnetic Resonance Spectroscopy
- Page ID
- 319871
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Spectroscopy is the study of the interaction of matter with light, or energy form the electromagnetic spectrum. Since we cannot visually observe the atoms and bonds in a molecule, we must use indirect techniques to determine a molecule’s identity. Spectroscopy allows us to infer clues into molecular structure based on the behavior of the molecules as they interact with different wavelengths of light. For example, microwave energy light causes molecules to tumble. Infrared wavelength light causes bonds to vibrate. Ultraviolet light causes electronic excitations. And on and on.
Nuclear magnetic resonance (NMR) spectroscopy is a tool that we use to elucidate molecular structure. It involves the absorption of low energy radiowaves. Radiowaves cause nuclear spin transitions, and NMR will detect what happens when this increase in energy is released in the presence of a magnetic field. This technology was the precursor for the development of the modern MRI machine.
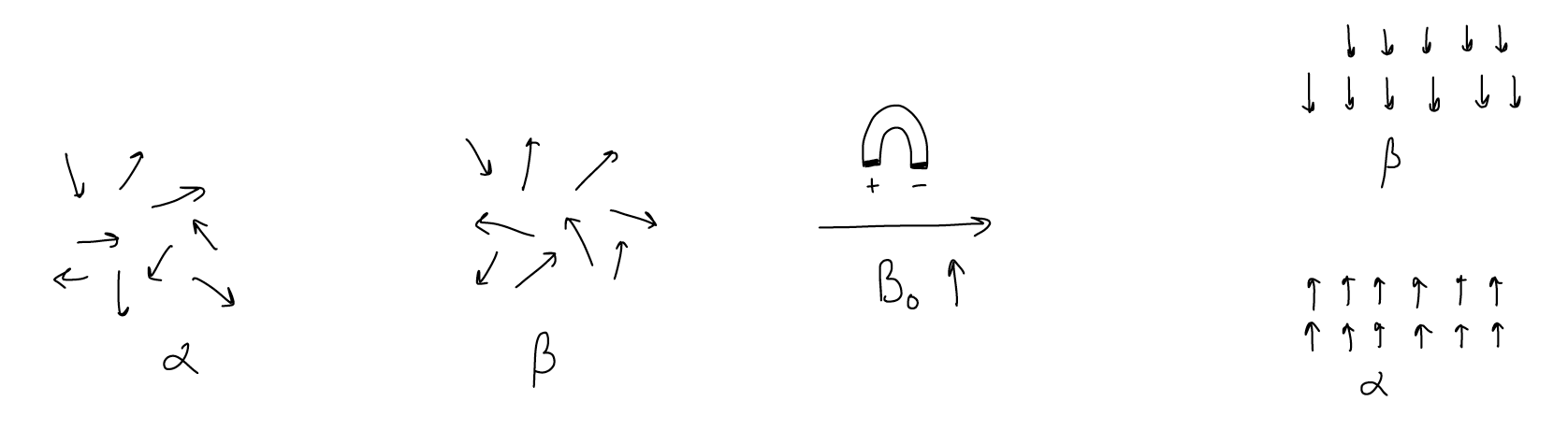
In order to understand why this occurs, it is important to review a little physics. Just like an electron, inherent to any nucleus is a spin quantum number known as “I.” I can be any integer, half-integer, or zero, but only nuclei that have non-zero spin quantum numbers are NMR-active (that is, we can see differences in their interaction with radiowaves). For all elements with even atomic numbers, I = 0. On the other hand, for the most useful nuclei in organic chemistry, we’ve luckily hit the jackpot - 1H and 13C nuclei have I = ½. For a given nucleus, the number of spin states is equal to 2I+1. So, for both 1H and 13C nuclei, the number of spin states is two: + ½ (or \(α\)) and – ½ (or \(β\)).
The most important feature of NMR is that in the absence of a magnetic field, all of the spin states are degenerate. The orientations of the spins are completely random and they are equal in energy. But since nuclei are themselves charged and moving, they possess angular momentum and thus generate a magnetic field. This causes the states to lose their degeneracy when a magnetic field is applied externally. Let’s look at this pictorially. When no magnetic field is present, the \(α\) and \(β\) states are random and equal in energy. When an external magnetic field (Bo) is applied, the \(α\) state becomes slightly lower in energy than the \(β\) state. Each of the spins aligns with the magnetic field for \(α\) or against the magnetic field for \(β\), and the number of spins in the \(α\) state is slightly greater than the \(β\) state (this is known as a Boltzmann distribution).
This is an important distinction and is the only reason why NMR works. If we were to plot energy versus the applied magnetic field, we see that as the magnetic field gets bigger, the difference between the \(α\) and \(β\) states gets bigger. This is known as Ziemann splitting. Absorption of the appropriate wavelength of light, then, will cause a spin to “flip” from the \(α\) state to the \(β\) state. For organic molecules, low energy radiowaves are energetic enough to promote this transition. The Larmour equation relates the frequency of this transition to the applied magnetic field:
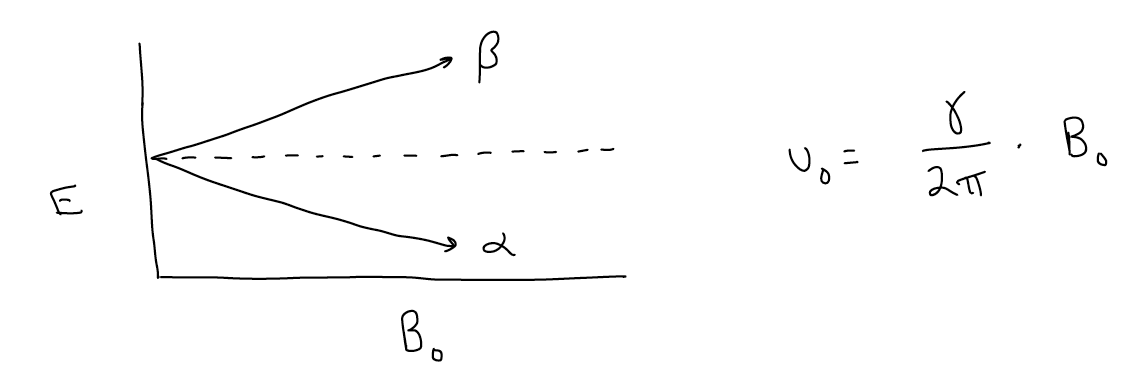
This frequency is different for each nucleus (conveniently for us, otherwise the signals would all overlap), with the distinguishing factor being the g value, which is the magnetogyric ratio, or how good a magnet a particular nucleus is. For example, the proton has a higher \(γ\) value than a carbon atom, so it’s Larmour frequency is also larger.
Let’s take a closer look at the Larmour equation and Ziemann splitting. Let’s say we were to use an NMR spectrometer that has a 70.5 kG magnet. The frequency of light needed to promote an \(α\) to \(β\) spin flip for a proton would be 75 MHz. But what if you take a spectrum at a higher or lower field instrument? Or, perhaps more likely, what if you want to compare your spectrum to a spectrum reported in the literature that was taken on a 60 kG machine. We know that as the magnetic field changes, the Larmour frequency also changes, so in order to compare spectra, we instead use a relative scale known as the d scale, or chemical shift. The \(δ\) scale is reported in parts per million and is equal to:
(\(ν\)i - \(ν\)TMS)/no x 106
where the shifts are all reported relative to tetramethylsilane (TMS). ni is the frequency of the desired peak and no is the idealized Larmour frequency. nTMS is obviously the frequency of the \(α\) to \(β\) spin flip for TMS. For the two most important nuclei, the chemical shift spectral width ranges from roughly 0-12 ppm for 1H and 0-220 ppm for 13C.