1.2: Using Chemical Arrows
- Page ID
- 319593
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The other common convention in organic chemistry is the use of arrows. Beware – not all arrows are created equal! Different types of arrows mean different things. Let’s go through each of them:
1.  This is a reaction arrow depicting conversion of one molecule to another: starting materials or substrates are drawn before the arrow; reagents and conditions are drawn above and below the arrow; products of the transformation are drawn to the right of the arrow.
This is a reaction arrow depicting conversion of one molecule to another: starting materials or substrates are drawn before the arrow; reagents and conditions are drawn above and below the arrow; products of the transformation are drawn to the right of the arrow.
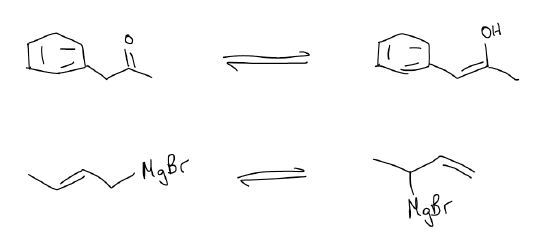
2.  This is an equilibrium arrow: two species on either side of the arrow are in equilibrium with each other, with a defined equilibrium constant (Keq). This does not mean that they are in equal amounts; it simply means that the rate of change between the two is constant. Equilibrium species involve the movement of atoms and involve bond breaking/bond forming events. This arrow designates that these species can be interconverted and that the forward direction is reversible. Some common example are below:
This is an equilibrium arrow: two species on either side of the arrow are in equilibrium with each other, with a defined equilibrium constant (Keq). This does not mean that they are in equal amounts; it simply means that the rate of change between the two is constant. Equilibrium species involve the movement of atoms and involve bond breaking/bond forming events. This arrow designates that these species can be interconverted and that the forward direction is reversible. Some common example are below:
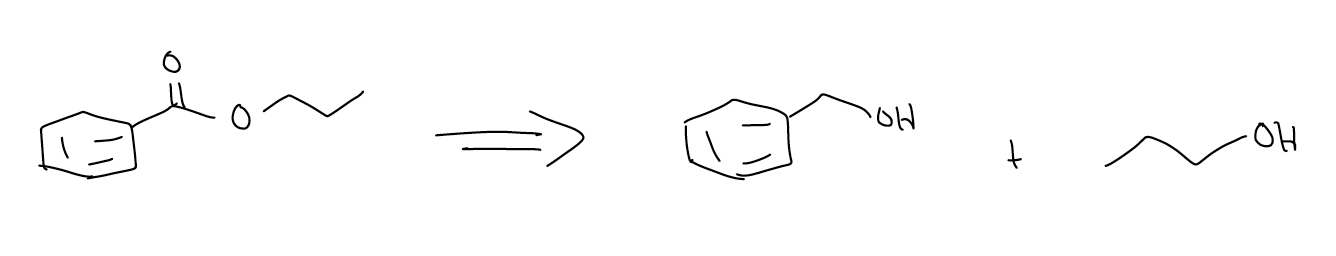
3.  This is a retrosynthetic arrow: the species before the arrow (normally more complicated) is synthesized from the species after the arrow (normally less complicated). The target molecule can be made by one or more steps. We use this type of arrow to plan syntheses of more complex molecules in the forward direction by detaching the more complex molecule in strategic ways.
This is a retrosynthetic arrow: the species before the arrow (normally more complicated) is synthesized from the species after the arrow (normally less complicated). The target molecule can be made by one or more steps. We use this type of arrow to plan syntheses of more complex molecules in the forward direction by detaching the more complex molecule in strategic ways.
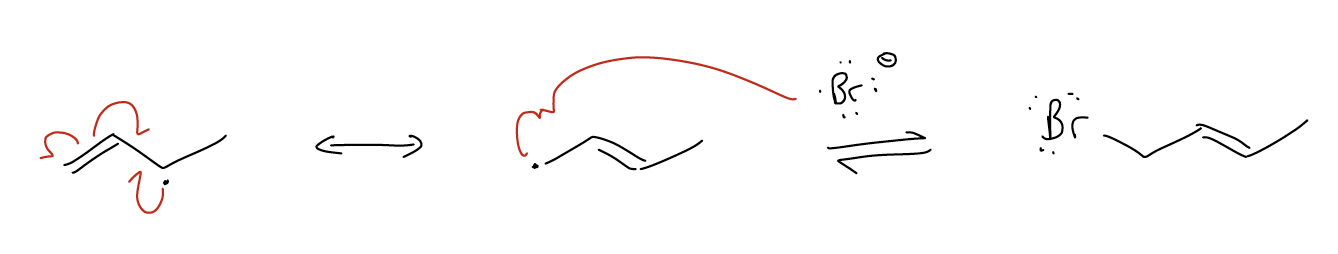
4.  This is a curved (curly) double-headed arrow: it is used to depict the movement of a pair of electrons from one place to another. It can be used to draw electron flow through a π system (a), or for electron flow in reactions (b). In the latter, known as a mechanism, arrows are always drawn from a pair of electrons (either a lone pair or bonding electrons) toward another atom/group.
This is a curved (curly) double-headed arrow: it is used to depict the movement of a pair of electrons from one place to another. It can be used to draw electron flow through a π system (a), or for electron flow in reactions (b). In the latter, known as a mechanism, arrows are always drawn from a pair of electrons (either a lone pair or bonding electrons) toward another atom/group.
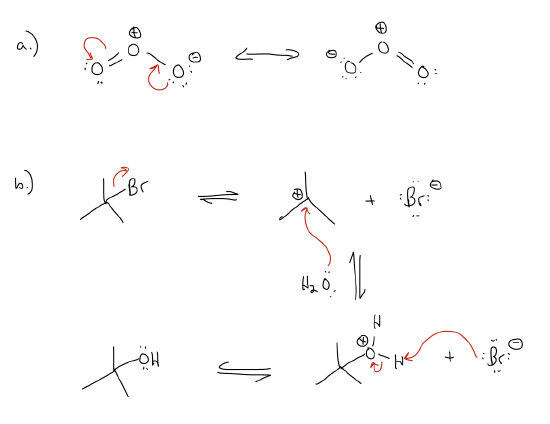
5.  This is a curved (curly) single-headed arrow: it is used to depict the movement of a single electron from one place to another, either in resonance or in a mechanism.
This is a curved (curly) single-headed arrow: it is used to depict the movement of a single electron from one place to another, either in resonance or in a mechanism.
6.  This is a dipole moment arrow: it depicts the direction of polarity, from partial positive charge to partial negative charge.
This is a dipole moment arrow: it depicts the direction of polarity, from partial positive charge to partial negative charge.

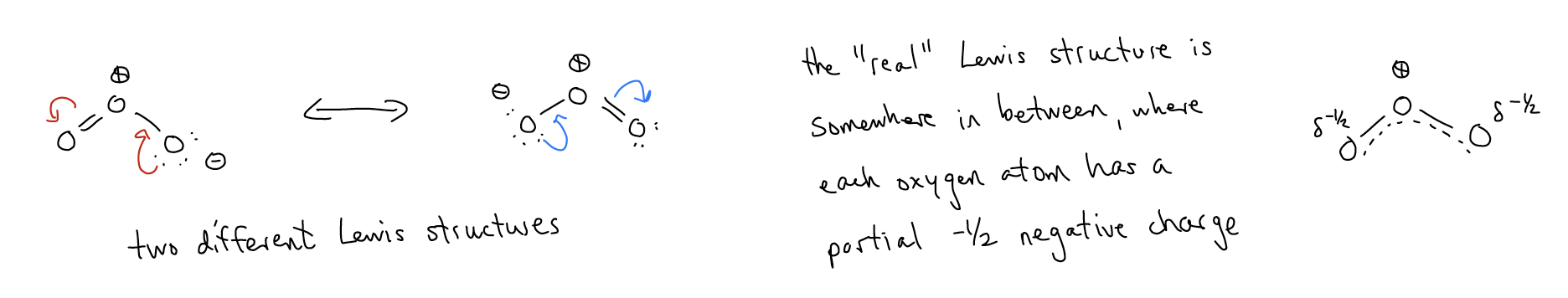
7.  This is a resonance arrow: it indicates that the real Lewis structure for a molecule is an average between the two. We use resonance arrows because our two-dimensional depiction of organic structures (that is, Lewis structures using line-angle notation) does not allow us to account for the movement of electrons through a \(π\) network. Take the example of ozone:
This is a resonance arrow: it indicates that the real Lewis structure for a molecule is an average between the two. We use resonance arrows because our two-dimensional depiction of organic structures (that is, Lewis structures using line-angle notation) does not allow us to account for the movement of electrons through a \(π\) network. Take the example of ozone: