1.1: Line Angle Notation as the Language of Chemical Structure
- Page ID
- 319590
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
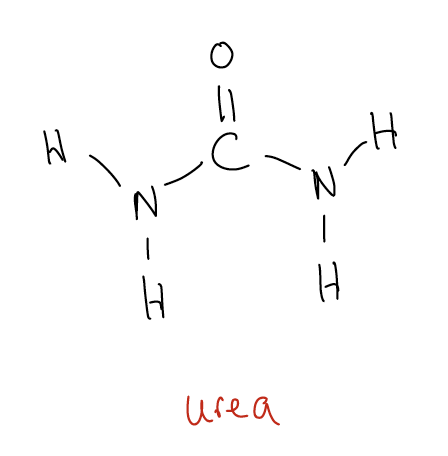
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Organic chemistry has often been defined as the chemistry of life or the chemistry of carbon-containing compounds. But this is actually a misnomer. The term “organic” emanated from the misconception that organic compounds were always related to life processes. We know now, however, that carbon-containing molecules can be produced by processes not involving living organisms. For example, molecules can be made from scratch in the research lab. Friedrich Wöhler was the first to synthesize an organic molecule (urea) from inorganic components. This was regarded as a true breakthrough in the field and provided direct evidence against the doctrine of vitalism – that living things are governed by different principles than non-living entities.
So, what is organic chemistry, then? After all, Wöhler’s synthesis of urea took place in 1828! A wise man once said that this particular branch of chemistry can be defined as “knowing how to make a molecule and knowing when you’ve made it.” I first want to introduce you to some conventions in the field because organic chemistry is a language. We communicate information about molecules and reactions in a certain way. Consider the molecule betulin, which is present in the bark of the white birch tree at a concentration as much as 30% by dry weight. We draw betulin, which is a small molecule with a molecular weight <500 g/mol, as such:
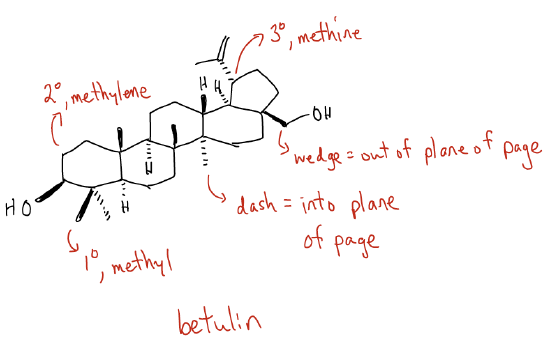
So, what does this all mean?
1. any corner of a polygon or branchpoint of any chain is a carbon atom.
2. no H atoms attached to C are drawn, unless they depict stereochemistry.
3. all H atoms attached to heteroatoms (ex. O, N, P, S, etc.) are shown (quick question: what kinds of heteroatoms are in betulin?).
4. one line connecting atoms is a single bond; two lines = double bond; three lines = triple bond (quick question: what kinds of bonds are in betulin?).
5. any end of a chain is a –CH3 group (quick question: how many CH3 groups in betulin?).
6. dashes and wedges indicate whether an atom or group is coming out of the plan of the page or into the plane of the page.
7. 1° (primary carbon) = one other carbon attached; 2° (secondary carbon) = two other carbons attached; 3° (tertiary) = three other carbons attached); 4° (quaternary) = four other carbons attached.
8. Methyl = CH3, Methylene = CH2, Methine = CH.
Given this depiction, one might think that organic molecules are flat – but don’t fall into this trap. Remember that carbon atoms have three different types of geometry:
sp = linear sp2 – trigonal planar sp3 – tetrahedral
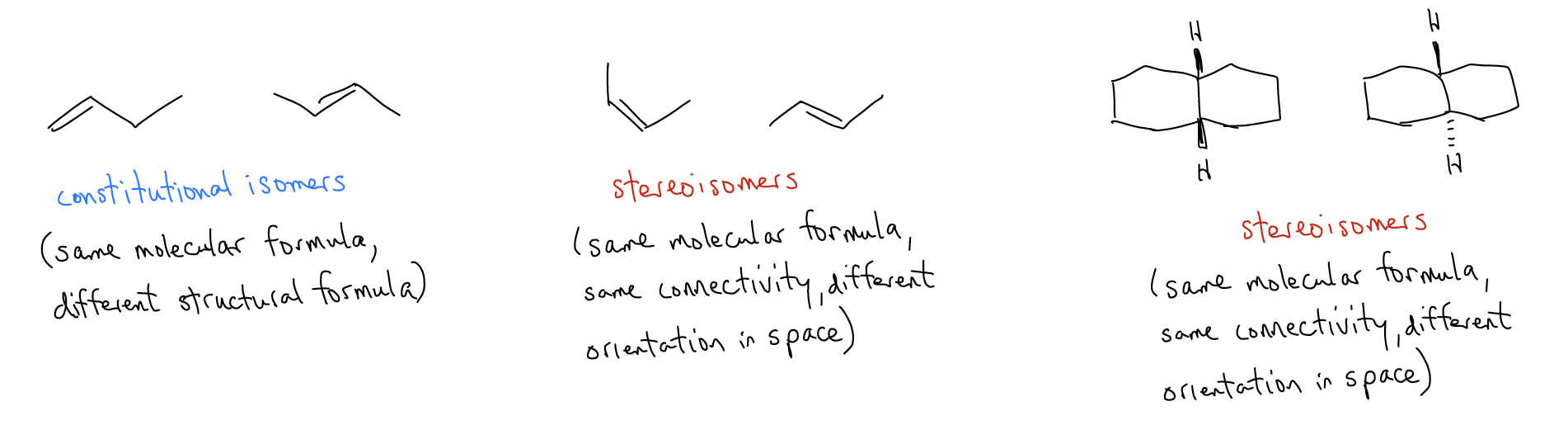
Only two of these geometries are flat – sp carbons are linear and rod-like and sp2 carbons are flat and trigonal. On the other hand, tetrahedral carbons have three-dimensionality, which means that the atoms or groups attached to carbon occupy a certain region in space. A specific orientation of atoms or groups in space is a molecular property known as stereochemistry. In betulin, this is depicted with a dash or wedge. Stereoisomers have the same constitution (same atoms connected to the same atoms), but different orientation in space. You may recall that constitutional isomers have the same molecular formula, but different structural formulas. Stereoisomers have the same molecular formula, same structural formulas (connectivity), but different orientation of atoms/groups in space.
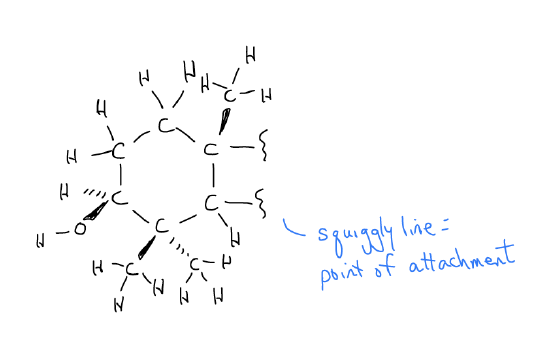
We call this shorthand for drawing organic molecules line-angle notation. Consider the alternative, that is if we were to draw out every single atom, as in the first ring of betulin shown below. Even with line-angle notation, we are limited, so if we really want insight into the structure of a molecule, we should build a model!
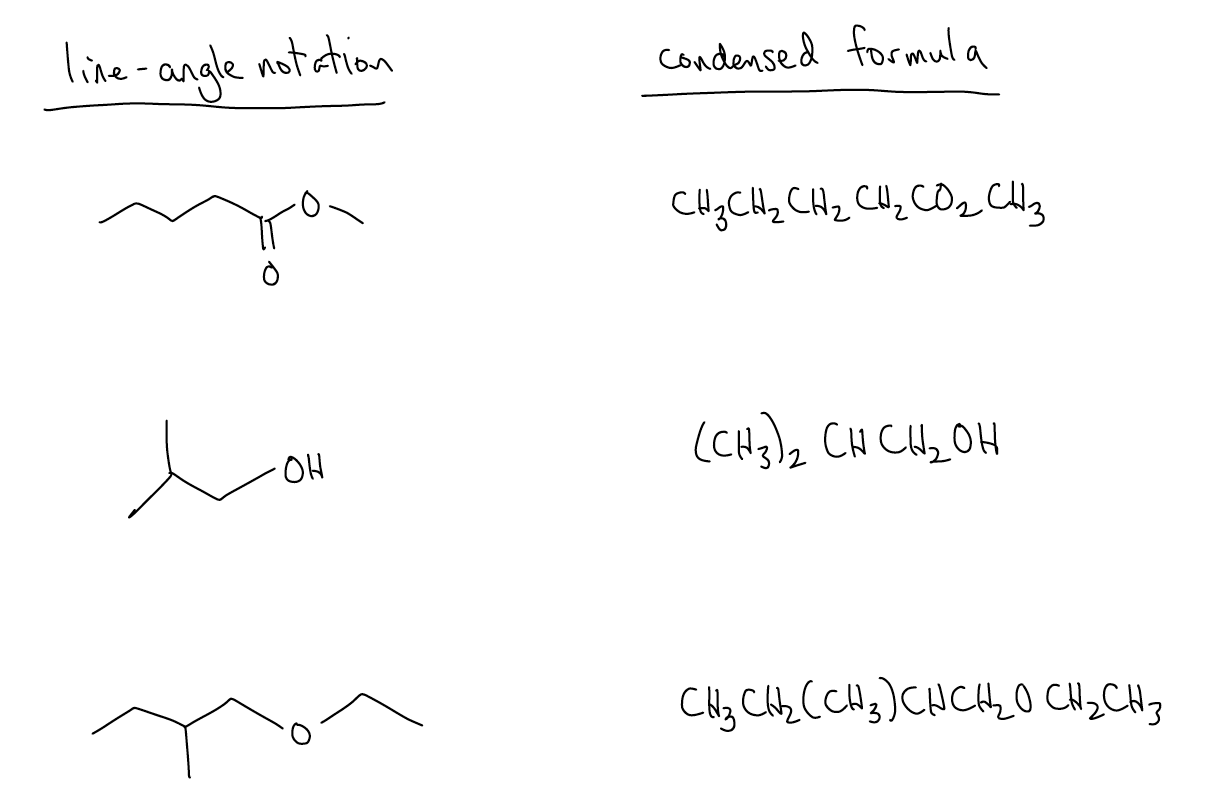
Line-angle notation can also be converted into condensed formulas in simple cases (does not apply for cyclic systems). Condensed formulas are “read” in line-angle structures from left to right carbon-by-carbon, taking into account heteroatoms and branching groups through the use of parentheses. For example:
Biomedical Spotlight
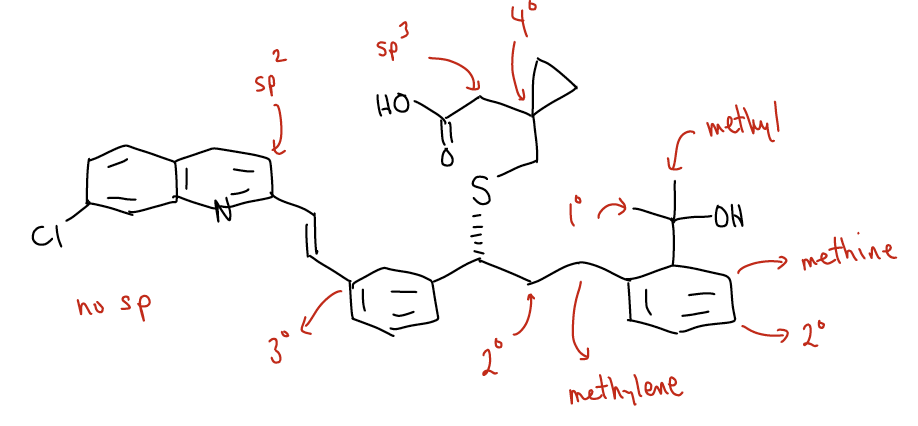
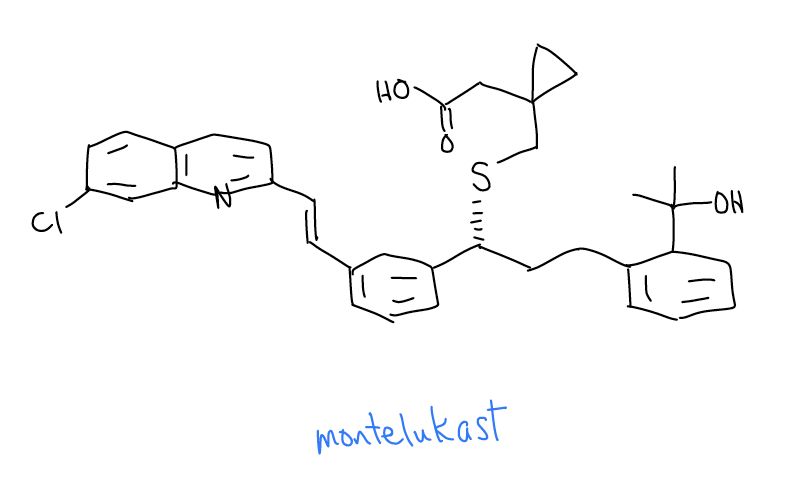
Montelukast is the active ingredient in the asthma drug that is sold under the brand name Singulair™. Its chemical structure shows the rich diversity in architecture of organic molecules. See if you can identify sp, sp2, sp3 carbon atoms, 1°, 2°, 3°, and 4° carbon atoms, and methyl, methylene, and methine C-H bonds.
Solution: