1.12: Ionization Energy
- Page ID
- 443921
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Estimated Time to Read: 4 min
Definition of Ionization Energy
Ionization energy is the energy required to remove an electron from a neutral atom in its gaseous phase. Ionization energy is always positive. Some elements can lose more than one electron and thus have several ionization energies. The first ionization energy is the energy required to remove the outermost, or highest energy, valence electron. The second ionization energy is the energy required to remove the next highest energy valence electron from a gaseous cation, etc. Below are the chemical equations describing the first and second ionization energies:
Equation \(\PageIndex{1}\): First Ionization Energy
\[ X_{(g)} \rightarrow X^+_{(g)} + e^- \nonumber \]
Equation \(\PageIndex{2}\): Second Ionization Energy
\[ X^+_{(g)} \rightarrow X^{2+}_{(g)} + e^- \nonumber \]
Table \(\PageIndex{1}\) gives the ionization energies for the period three elements. Notice how the second ionization energy for sodium, third ionization energy for magnesium, fourth ionization energy for aluminum, etc. are all much greater than the preceding ionization energy. This occurs because the preceding configuration was in a stable octet formation; therefore a much larger amount of energy is required to ionize the valence electron and break the octet formation.
| Element | 1st | 2nd | 3rd | 4th | 5th | 6th | 7th |
|---|---|---|---|---|---|---|---|
| Na | 496 | 4562 | |||||
| Mg | 738 | 1451 | 7733 | ||||
| Al | 577 | 1817 | 2745 | 11580 | |||
| Si | 786 | 1577 | 3232 | 4356 | 16090 | ||
| P | 1060 | 1903 | 2912 | 4957 | 6274 | 21270 | |
| S | 999.6 | 2251 | 3361 | 4564 | 7013 | 8496 | 27110 |
| Cl | 1256 | 2297 | 3822 | 5158 | 6542 | 9362 | 11020 |
| Ar | 1520 | 2666 | 3931 | 5771 | 7238 | 8781 | 12000 |
Trends in Ionization Energy
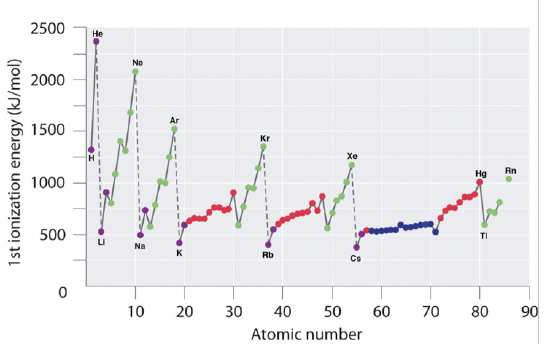
The lower the ionization energy is, the more readily the atom becomes a cation. Therefore, the higher the ionization energy is, the less readily the atom becomes a cation (Figure \(\PageIndex{1}\)). Generally, elements on the right side of the periodic table have a higher ionization energy because their valence shell is nearly filled. The highest ionization energies are the noble gases because they all have high effective charge due to their octet formation. Elements on the left side of the periodic table have low ionization energies because losing an electron allows them to have the noble gas configuration. Therefore, it requires less energy to remove one of their valence electrons, and ionization energy increases from left to right on the periodic table.
This trend can also be explained by the fact that the effective nuclear charge increases from the left to the right within a period as the number of protons increases. With each addition of a proton, the pull of the nucleus on the outmost electron increases, and it becomes more difficult to overcome the attractive forces of the nucleus and remove this electron from the atom.
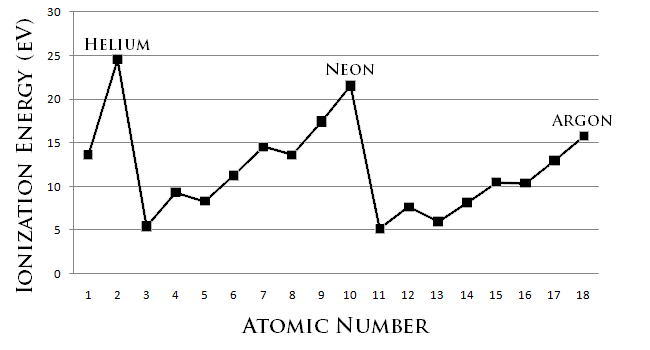
Within the main group elements, the trend is broken two times (Figure \(\PageIndex{2}\)). First, the group 13 elements have a lower ionization energy than the group 2 elements. Second, the group 16 elements have a lower ionization energy than the group 15 elements. This is explained by the fact that the filled and half-filled subshells in group 2 and group 15, respectively, are particularly stable electron configurations; thus, the electrons within these subshells have unusually low energy and require greater energy to be removed from the atom.
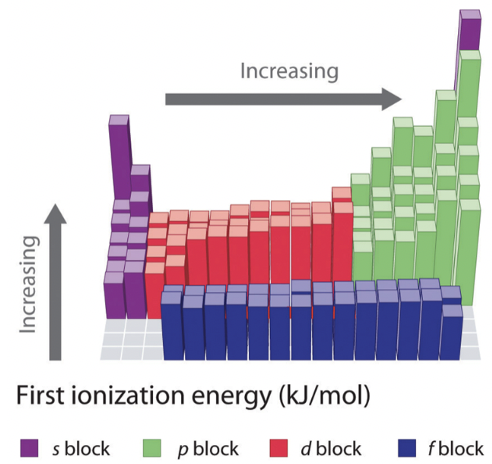
The increase in ionization energy across the period is less pronounced within the d-block, and even less pronounced within the f-block. This is because the effective nuclear charge on the outmost electrons does not increase as much because inner d and f orbitals are getting filled. Following the d-block, group 13 elements have a lower ionization energy than the group 12 elements. This is because in group 13 electrons are added to a new p subshell.
Ionization energy becomes smaller down a group as electrons are added to higher energy shells and the distance from the nucleus increases (n increases). The attractive forces of the nucleus are diminished as the electrons are further from the nucleus.
Generally, any subsequent ionization energies (2nd, 3rd, etc.) follow the same periodic trend as the first ionization energy.

- The ionization energy of the elements within a period generally increases from left to right. This is due to valence shell stability.
- The ionization energy of the elements within a group generally decreases from top to bottom. This is due to increasing electron energy shell.
- The noble gases possess very high ionization energies because of their full valence shells.
Problems
Based on the periodic trends for ionization energy, which element has the highest ionization energy?
- Fluorine (F)
- Nitrogen (N)
- Helium (He)
- Answer
-
c. Helium (He)
Which reaction do you expect to occur?
- \(\ce{I2(g) + 2Br (aq) → Br2 (s) + 2I(aq)}\)
- \(\ce{Cl2(g) + 2I (aq) → I2 (s) + 2Cl(aq)}\)
- Answer
-
a.
Based on the periodic trends for ionization energy, which element has the highest ionization energy?
- Answer
-
Helium,He
References
- Cotton, F.A.; Wilkinson, G. (1988). Advanced Inorganic Chemistry (5th Edn). New York: Wiley. ISBN 0-471-84997p. 1385.
- Hutchinson, John. "Journal of Chemical Education." Concept Development Studies in Chemistry (2007). Print. Outside Links
- Jolly, William L. (1991). Modern Inorganic Chemistry (2nd Edn.). New York: McGraw-Hill. ISBN 0-07-112651-1.
- Petrucci, Ralph H. General Chemistry. 9th ed. New Jersey: Pearson Prentice Hall, 2005.

