1.13: Electron Affinity
- Page ID
- 443922
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Estimated Time to Read: 4 min
Definition of Electron Affinity
Electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral gaseous atom. The more negative the electron affinity value, the higher the electron affinity and the more easily an electron is added to an atom. Electron affinity can be either positive or negative. The greater the negative value, the more stable the anion is.
Equation \(\PageIndex{1}\): Exothermic, positive electron affinity
\[\ce{X(g) + e^{-} -> X^{-} + Energy} \nonumber \]
Equation \(\PageIndex{2}\): Endothermic, negative electron affinity
\[\ce{X(g) + e^{-} + Energy -> X^{-}} \nonumber \]

Figure \(\PageIndex{1}\) shows that most elements have negative electron affinities, meaning that the addition of a free electron to a neutral atom is exothermic. However, this is not always the case. Some elements (the noble gases, Be, N, Mg, Zn, Cd, and Hg) have positive electron affinities. Zn, Cd, and Hg are group 12 elements with a full d subshell, which is particularly stable, and an additional electron would need to be added to a p orbital of a higher shell which is energetically unfavorable. Similarly, Be and Mg have filled s subshells, which are also stable, and adding an additional electron would require a new subshell, which is energetically unfavorable. Ca and Sr have electron affinities of about 0, and that of Ba is only slightly negative. This shows that higher periods make the addition of an electron to a new p subshell slightly more favorable. Nitrogen has a half-filled p subshell, which is also a quite stable electron configuration, and therefore, adding an electron is not favorable. The addition of an electron is slightly more favorable for the other group 15 elements, however.
Trends in Electron Affinity
Moving from left to right across a period, atoms become smaller as the nuclear charge and the attractive forces of the nucleus become stronger. Electrons added to the valence shell are stabilized by the attractive force of the nucleus, thus the electron affinity increases (becomes more negative) more from left to right across a period. Electron affinity generally decreases (becomes less negative) down a group of elements as the distance from the nucleus of valence electrons increases as electrons are added to higher energy shells (n increases). The attractive forces of the nucleus decrease with a larger distance between the negatively-charged electron and the positively-charged nucleus, and therefore, electron affinity decreases.
Generally, the elements on the right side of the periodic table will have large negative electron affinity. However, Nitrogen, Oxygen, and Fluorine do not follow this trend. The noble gas electron configuration will be close to zero because they will not easily gain electrons. The general trend is also broken for group 2 and group 15 elements because these elements have full and half-filled subshells, respectively. In addition, group 1 elements have higher electron affinities than group 3 elements. This is because adding an electron to a group 1 element produces a full s subshell, which is fairly stable. For the d-block elements, the electron affinity tends to increase from group 3 to group 11, but the trend is broken multiple times and each element would need to be investigated individually. There is a large drop in electron affinity from group 17 to group 18 which is explained by the fact that the addition of an electron to a group 17 element produces a filled shell, while a group 18 element already has a full shell and the addition of an electron would require a new shell. Similarly, there is a sharp drop in electron affinity from group 11 to group 12, as an addition of an electron to a group 11 atom produces a full subshell, while the addition of an electron to a group 12 element would require the start of new subshell.
In general, electron affinity decreases down a group. For the s-block there is a steady decrease down a group.For the p block elements the electron affinity tends to first increase from period 2 to 3, and then decreases. For d block elements there is no clear trend for the electron affinity.
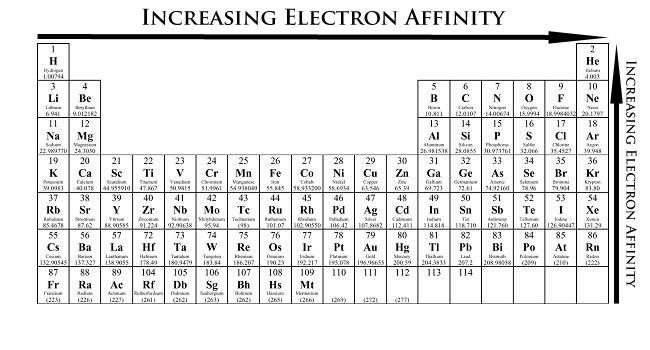
- Electron affinity increases (becomes more negative) from left to right within a period. This is caused by the decrease in atomic radius.
- Electron affinity decreases (becomes less negative) from top to bottom within a group. This is caused by the increase in atomic radius.
Problems
Arrange the following elements in order of decreasing electron affinity: fluorine (F), phosphorous (P), sulfur (S), boron (B).
- Answer
-
F > S > P > B
Arrange these elements according to increasingly negative electron affinity: Ba, F, Si, Ca, O .
- Answer
-
Ba, Ca, Si, O, F
The following series of problems reviews general understanding of the aforementioned material.
Rewrite the following list in order of decreasing electron affinity: fluorine (F), phosphorous (P), sulfur (S), boron (B).
- Answer
-
Fluorine (F)>Sulfur (S)>Phosphorous (P)>Boron (B). Explanation: Electron affinity generally increases from left to right and from bottom to top.

