1.11: Atomic Radius
- Page ID
- 443677
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\(\newcommand{\ket}[1]{\left| #1 \right>} \)
\( \newcommand{\bra}[1]{\left< #1 \right|} \)
\( \newcommand{\braket}[2]{\left< #1 \vphantom{#2} \right| \left. #2 \vphantom{#1} \right>} \)
\( \newcommand{\qmvec}[1]{\mathbf{\vec{#1}}} \)
\( \newcommand{\op}[1]{\hat{\mathbf{#1}}}\)
\( \newcommand{\expect}[1]{\langle #1 \rangle}\)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Estimated Time to Read: 6 min
Determining Atomic Radius
The atomic radius is one-half the distance between the nuclei of two atoms (just like a radius is half the diameter of a circle). However, this idea is complicated by the fact that not all atoms are normally bound together in the same way. Some are bound by covalent bonds in molecules, some are attracted to each other in ionic crystals, and others are held in metallic crystals. The SI units for measuring atomic radii are the nanometer (nm) and the picometer (pm). \(1 \, nm = 1 \times 10^{-9}\, m\) and \(1\, pm = 1 \times 10^{-12}\, m\).
Atomic radii can be obtained from quantum mechanical calculations or can be determined experimentally. When calculated, the atomic radius is defined as the radius of the spherical volume in which the electron can be observed with 90% probability. Calculate atomic radii are shown in Figure \(\PageIndex{1}\).
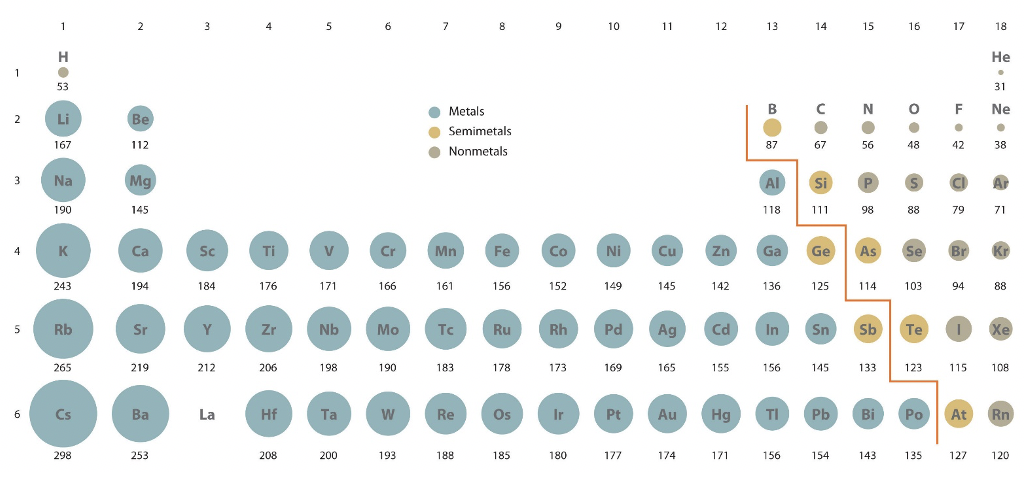
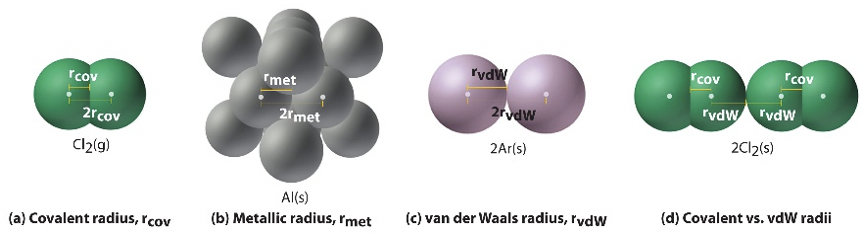
The measurement of atomic or ionic size will depend on a number of factors, including the covalent character of bonding in any particular molecule, coordination number, physical state (liquid, solid, gas), the identity of nearby atoms/ions, variation in crystal structure, and distortions within regular crystal structures. You should keep in mind that the size of an atom or ion is a "fuzzy" measure, and the radius under a different set of conditions will probably change slightly. Depending on how the atomic radius is measured, it will be classified as a (Figure \(\PageIndex{2}\)):
- Covalent radius: The radius of an atom is derived from the bond length within a nonpolar molecule; one-half the distance between the nuclei of two atoms within a covalent bond.
- Metallic or Crystal radius: The radius of an atom is determined using electron density maps from X-ray data; one-half the distance between the nuclei of two neighboring atoms in a crystal structure.
- van der Waals radius: The radius of an atom is determined by collision with other atoms; one-half the distance between two atoms that are not bonded but are brought together by intermolecular forces.
The relative atomic sizes shown in Figure \(\PageIndex{3}\) were derived from crystallographic data.
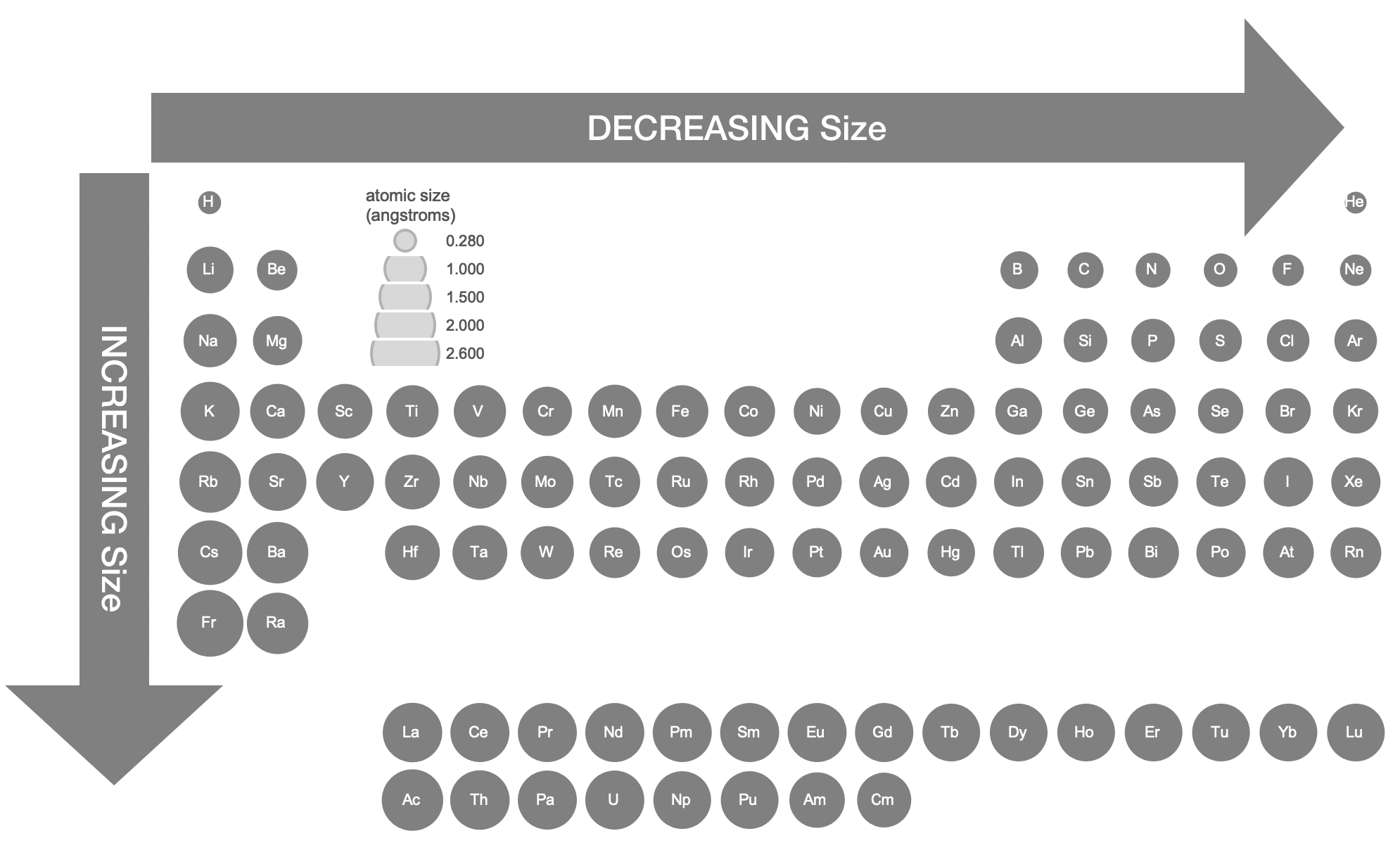
Trends in Atomic Radius
Moving from left to right across a period, a proton is added to the nucleus for each element, making the nucleus more positively charged. At the same time, an electron is added the the electron energy shell, adding negative charge. The effect of increasing the number of protons is greater than that of increasing the number of electrons; therefore, there nuclear attraction for the electrons increases with the addition of each proton and electron. This means that the nucleus attracts the electrons more strongly, pulling the atom's shell closer to the nucleus. As a result, the atomic radius decreases as you move left to right across a period (Figure \(\PageIndex{4}\)).
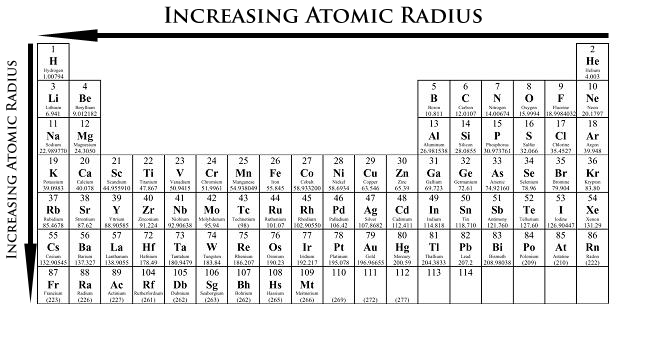
Down a group, atomic radius increases. The valence electrons occupy higher energy shells due to the increasing quantum number (n). As a result, the valence electrons are further away from the nucleus as n increases.
- Atomic radius decreases from left to right within a period. This is caused by the increase in the number of protons and electrons across a period. One proton has a greater effect than one electron; thus, electrons are pulled towards the nucleus, resulting in a smaller radius.
- Atomic radius increases from top to bottom within a group. This is caused by electrons being add to higher energy levels that are futher from the nucleus.
Trends in Ionic Radius
An ionic radius is one-half the distance between the nuclei of two ions in an ionic bond. The distance must be apportioned for the smaller cation and larger anion. Trends in ionic radius follow the general trends in atomic radius for ions of the same charge. Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration.
Cations
Compared to their atoms, cations have the same number of protons but fewer electrons. Removal of electrons from an atom to form a cation results in a significant increase in effective nuclear charge, resulting in all other electrons being more strongly attracted to the nucleus. The result is a contraction in size from the atom to cation. Figure \(\PageIndex{5}\) visually illustrates the relative size of atoms and some cations of the first four periods; the data is available in tabular format in Figure \(\PageIndex{6}\).
Anions
Compared to their atoms, anions have the same number of protons but more electrons. Addition of electrons to an atom to form an anion results in a decrease in effective nuclear charge, which corresponds to a decrease in attractive force between the nucleus and electrons. Lower attractive force leads to expansion, where the size of the atom becomes larger in the formation of an anion. Figure \(\PageIndex{5}\) visually illustrates the relative size of atoms and some anions of the first four periods, while the data is available in tabular format in Figure \(\PageIndex{6}\).
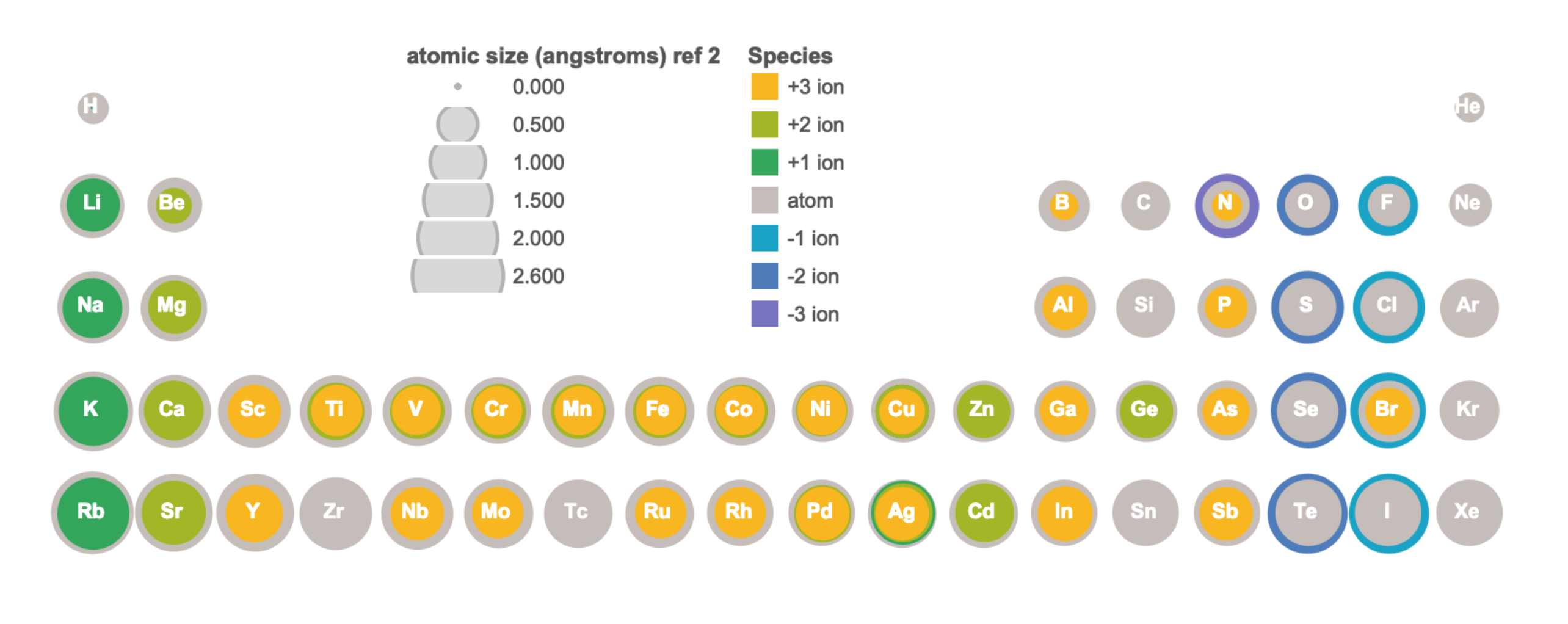
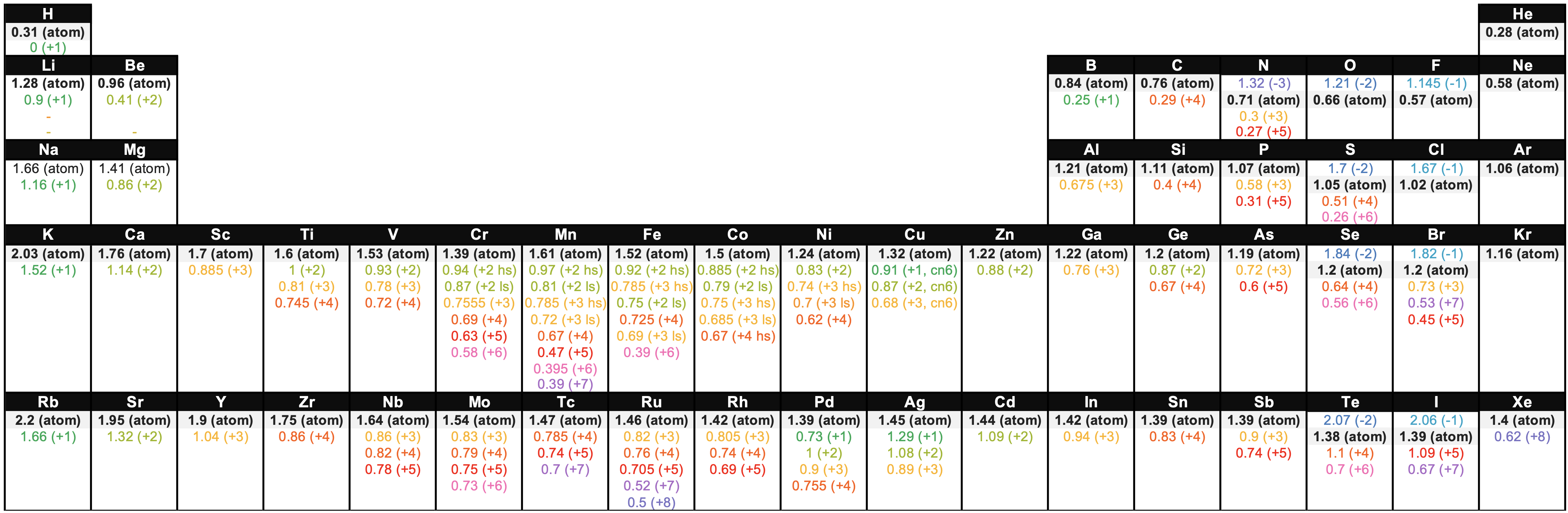
Figure \(\PageIndex{6}\). Atomic and ionic radii in Angstroms of elements in the first four periods of the periodic table. Radii from each element are listed from largest to smallest. Ionic charge is indicated in parentheses. (hs = high spin, ls = low spin, cn6 = coordination number is 6).1-3 (ChemLibre)
Problems
True or False? Nitrogen has a larger atomic radius than oxygen.
- Answer
-
True
Atomic radius increases from right to left on the periodic table. Therefore, nitrogen is larger than oxygen.
Which atom has a smaller atomic radius smaller than that of sulfur (S)?
- Oxygen (O)
- Chlorine (Cl)
- Calcium (Ca)
- Lithium (Li)
- None of the above
- Answer
-
a. Oxygen (O)
Periodic trends indicate that atomic radius increases up a group and from left to right across a period. Therefore, oxygen has a smaller atomic radius sulfur
True or False? A nonmetal has a smaller ionic radius compared with a metal of the same period.
- Answer
-
False
The reasoning behind this lies in the fact that a metal usually loses an electron in becoming an ion while a non-metal gains an electron. This results in a smaller ionic radius for the metal ion and a larger ionic radius for the non-metal ion.
Arrange these elements according to decreasing atomic size: Na, C, Sr, Cu, Fr
- Answer
-
Fr, Sr, Cu, Na, C
Which has a larger atomic radius: nitrogen or oxygen?
- Answer
-
Atomic radius increases from right to left on the periodic table. Therefore, nitrogen is larger than oxygen
Which of these elements has a smaller atomic radius than sulfur (S): O, Cl, Ca, Li
- Answer
-
Oxygen (O) is the only element in the list with a smaller atomic radius than S. Periodic trends indicate that atomic radius increases down a group (from top to bottom) and from left to right across a period.
References
- Cordero, Beatriz, Veronica Gomez, Ana E. Platero-Prats, Marc Reves, Jorge Echeverria, Eduard Cremades, Flavia Barragan, and Santiago Alvarez. “Covalent Radii Revisited.” Dalton Transactions, no. 21 (2008): 2832–38. doi:10.1039/b801115j.
- R. D. Shannon (1976). "Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides". Acta Crystallogr A. 32 (5): 751–767. Bibcode:1976AcCrA..32..751S. doi:10.1107/S0567739476001551.
- Wikipedia articles on Atomic Radius and Ionic Radius.

