1.10: The Periodic Table and Periodic Properties
- Page ID
- 454043
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Estimated Time to Read: 5 min
The Periodic Table
The Periodic Table of Elements categorizes like elements together. Dmitri Mendeleev, a Russian scientist, was the first to create a widely accepted arrangement of the elements in 1869. Mendeleev believed that when the elements are arranged in order of increasing atomic mass, certain sets of properties recur periodically. Although most modern periodic tables are arranged in eighteen groups (columns) of elements, Mendeleev's original periodic table had the elements organized into eight groups and twelve periods (rows).
On the periodic table, elements that have similar properties are in the same groups (vertical). From left to right, the atomic number (z) of the elements increases from one period to the next (horizontal). The groups are numbered at the top of each column and the periods on the left next to each row. The main group elements are groups 1,2 and 13 through 18. These groups contain the most naturally abundant elements, and are the most important for life. The elements shaded in light pink in the table above are known as transition metals. The two rows of elements starting at z=58, are sometimes called inner transition metals and have that have been extracted and placed at the bottom of the table, because they would make the table too wide if kept continuous. The 14 elements following lanthanum (z=57) are called lanthanides, and the 14 following actinium (z=89) are called actinides.
Elements in the periodic table can be placed into two broad categories, metals and nonmetals. Most metals are good conductors of heat and electricity, are malleable and ductile, and are moderate to high melting points. In general, nonmetals are nonconductors of heat and electricity, are nonmalleable solids, and many are gases at room temperature. Just as shown in the table above, metals and nonmetals on the periodic table are often separated by a stairstep diagonal line, and several elements near this line are often called metalloids (Si, Ge, As, Sb, Te, and At). Metalloids are elements that look like metals and in some ways behave like metals but also have some nonmetallic properties. The group to the farthest right of the table, shaded orange, is known as the noble gases. Noble gases are treated as a special group of nonmetals.
Alkali Metals/Alkali Earth Metals
The Alkali metals are comprised of group 1 of the periodic table and consist of Lithium, Sodium, Rubidium, Cesium, and Francium. These metals are highly reactive and form ionic compounds (when a nonmetal and a metal come together) as well as many other compounds. Alkali metals all have a charge of +1 and have the largest atom sizes than any of the other elements on each of their respective periods.
Alkali Earth Metals are located in group 2 and consist of Beryllium, Magnesium, Calcium, Strontium, Barium, and Radium. Unlike the Alkali metals, the earth metals have a smaller atom size and are not as reactive. These metals may also form ionic and other compounds and have a charge of +2.
Transition Metals
The transition metals range from groups IIIB to XIIB on the periodic table. These metals form positively charged ions, are very hard, and have very high melting and boiling points. Transition metals are also good conductors of electricity and are malleable.
Lanthanides and Actinides
Lanthanides (shown in row ** in chart above) and Actinides (shown in row * in chart above), form the block of two rows that are placed at the bottom of the periodic table for space issues. These are also considered to be transition metals. Lanthanides are form the top row of this block and are very soft metals with high boiling and melting points. Actinides form the bottom row and are radioactive. They also form compounds with most nonmetals. To find out why these elements have their own section, check out the electron configurations page.
Metalloids
Metalloids are located along the staircase separating the metals from the nonmetals on the periodic table. Boron, silicon, germanium, arsenic, antimony, and tellurium all have metal and nonmetal properties. For example, Silicon has a metallic luster but is brittle and is an inefficient conductor of electricity like a nonmetal. As the metalloids have a combination of both metallic and nonmetal characteristics, they are intermediate conductors of electricity or "semiconductors".
Halogens
Halogens are comprised of the five nonmetal elements Flourine, Chlorine, Bromine, Iodine, and Astatine. They are located on group 17 of the periodic table and have a charge of -1. The term "halogen" means "salt-former" and compounds that contain one of the halogens are salts. The physical properties of halogens vary significantly as they can exist as solids, liquids, and gases at room temperature. However in general, halogens are very reactive, especially with the alkali metals and earth metals of groups 1 and 2 with which they form ionic compounds.
Noble Gases
The noble gases consist of group 18 (sometimes reffered to as group O) of the periodic table of elements. The noble gases have very low boiling and melting points and are all gases at room temperature. They are also very nonreactive as they already have a full valence shell with 8 electrons. Therefore, the noble gases have little tendency to lose or gain electrons.
A Summary of Periodic Properties
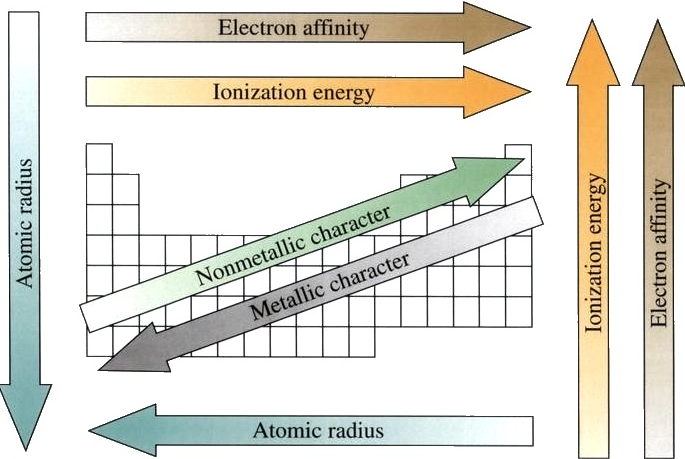
Problems
Which element is an example of a metalloid
- S
- Zn
- Ge
- Re
- none of these
- Answer
-
c. Ge
In the periodic table, the vertical (up and down) columns are called
- periods
- transitions
- families/groups
- metalloids
- none of these
- Answer
-
c. families/groups
Why are noble gases inert (nonreactive)?
- Answer
-
Noble gases are inert because they already have a full valence electron shell and have little tendency to gain or lose electrons.
Lanthanides and Actinides are
- alkali earth metals
- transition metals
- metalloids
- alkali metals
- none of these
- Answer
-
b. transtion metals

