1.9: Electron Configurations for Transition Metal Elements
- Page ID
- 443911
Estimated Time to Read: 6 min
Basic Steps
Writing an electron configuration for a transition metal element follows the same basic steps as for writing an electron configuration for an element in the s-block or p-block. List each subshell, and then fill each subshell with an appropriate number of electrons until all electrons in the element are accounted for. Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations. First, recall that the n = 3 shell is the first shell to have a d subshell. Second, recall that the 4s subshell is lower in energy than the 3d subshell. Thus, when filling electrons into orbitals the correct order of filling is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6. Similarly, the 5s will be filled before the 4d, and the 6s will be filled before the 5d.
Some elements in the d block will also have electrons in the f subshell. The n = 4 shell is the first shell to have a f subshell. Thus, the 6s subshell is lower in energy than the 4f subshell, and the correct order of filling is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6. Similarly, the 7s will be filled before the 5f, which will be filled before the 6d subshell.
Write the ground state electron configuration for vanadium (V).
Solution: Method 1
- Find the number of electrons in the atom: Vanadium has 23 electrons.
- List the subshells following the model shown in Figure 1.6.3: 1s 2s 2p 3s 3p 4s 3d
- Fill the subshells until all electrons have been accounted for. Remember: each s subshell can hold 2 electrons, each p subshell can hold six electrons, each d subshell can hold 10 electrons, and each f subshell can hold 14 electrons: 1s2 2s2 2p6 3s2 3p6 4s2 3d3
Solution: Method 2
- Locate the atom on the periodic table.
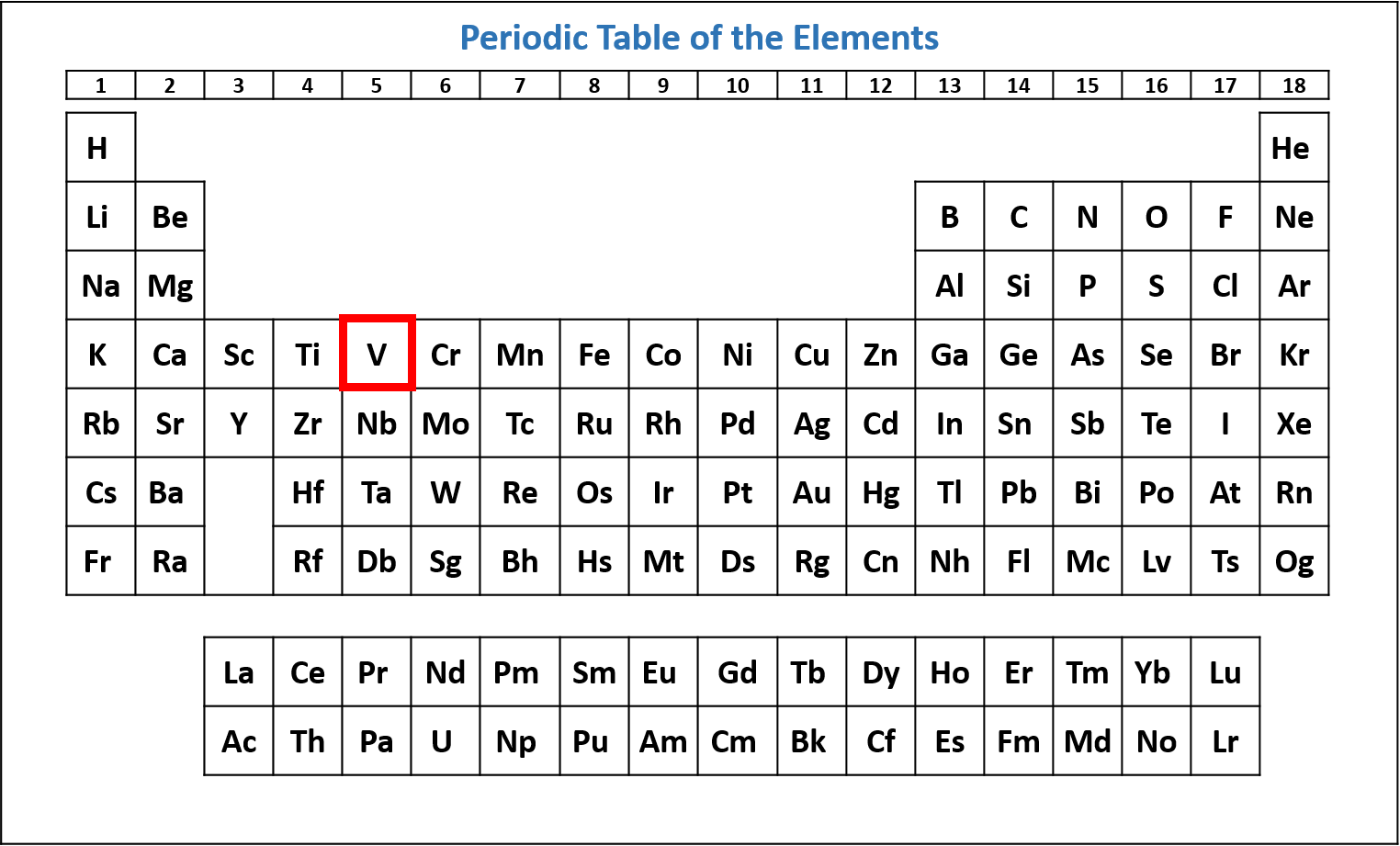
Figure \(\PageIndex{1}\): Periodic table of the elements with the location of vanadium (V) highlighted. (CC-BY-NC-SA; Kathryn A. Newton) - Starting at hydrogen and the 1s subshell, read across each row of the periodic table until you get to your chosen element. As you read across each row, list each subshell and the number of electrons in the subshell.
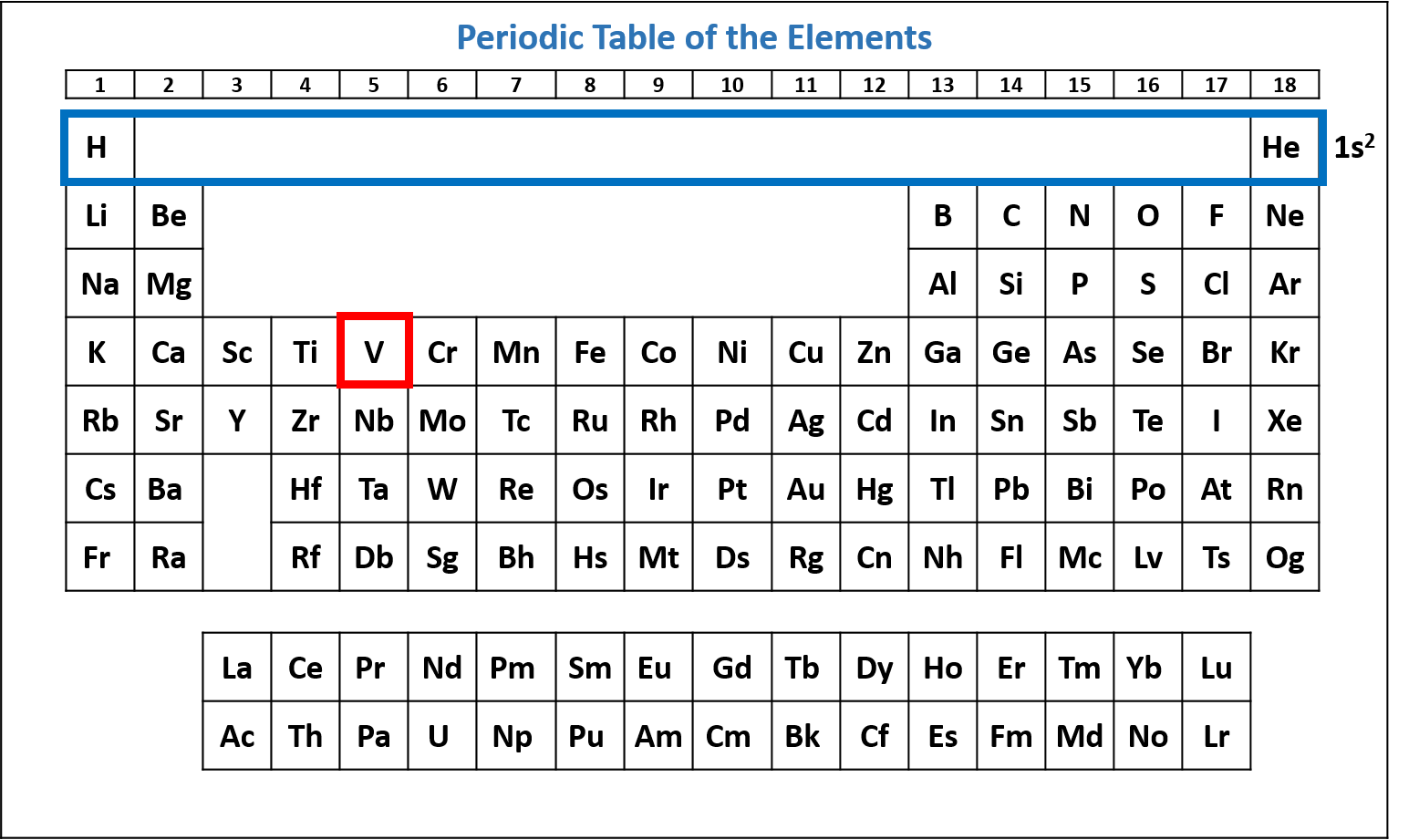
Figure \(\PageIndex{2}\): Periodic table of the elements with the 1s2 subshell highlighted. V has two electrons in the 1s subshell. (CC-BY-NC-SA; Kathryn A. Newton) 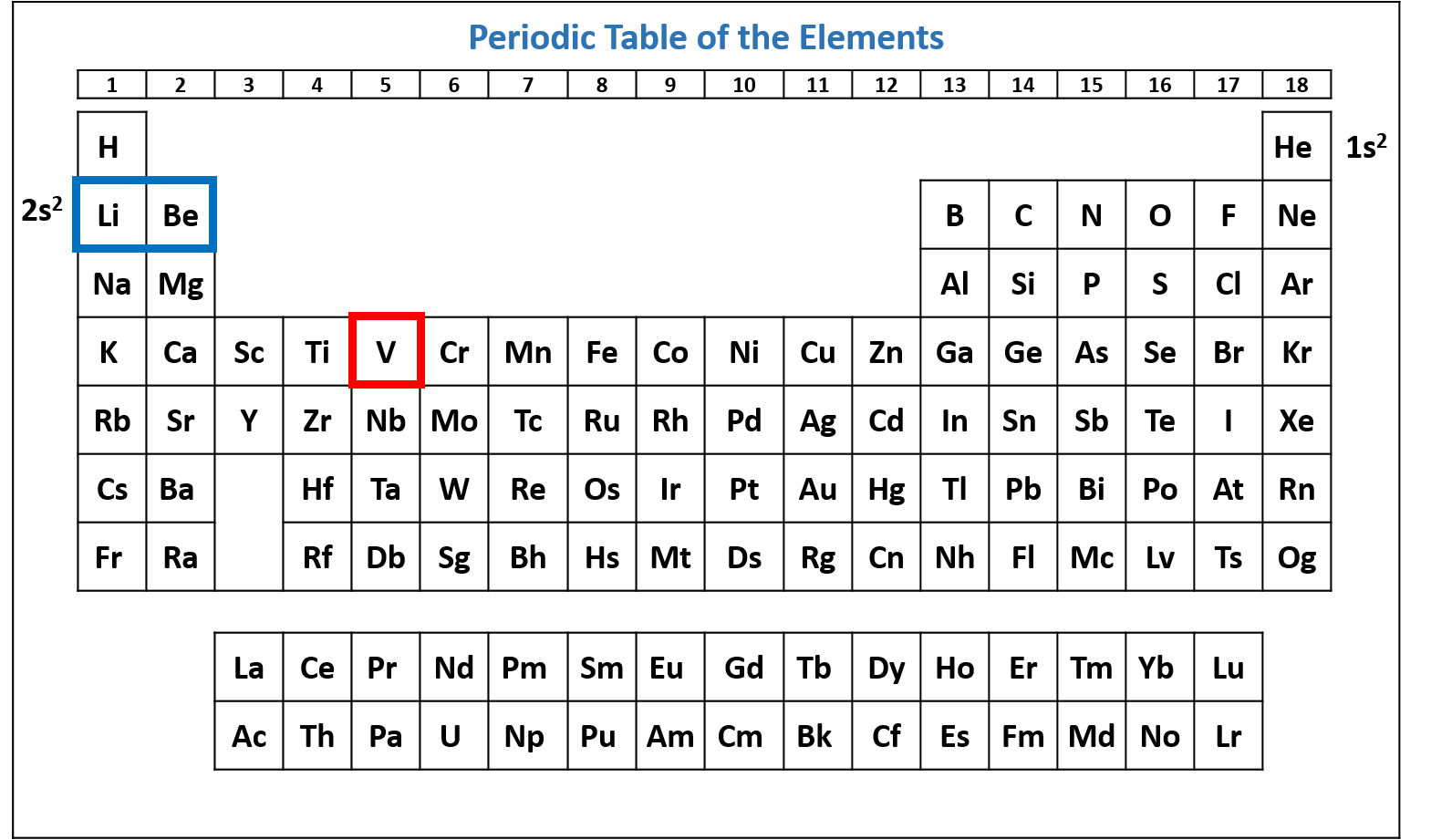
Figure \(\PageIndex{3}\): Periodic table of the elements with the 2s2 subshell highlighted. V has two electrons in the 2s subshell. (CC-BY-NC-SA; Kathryn A. Newton) 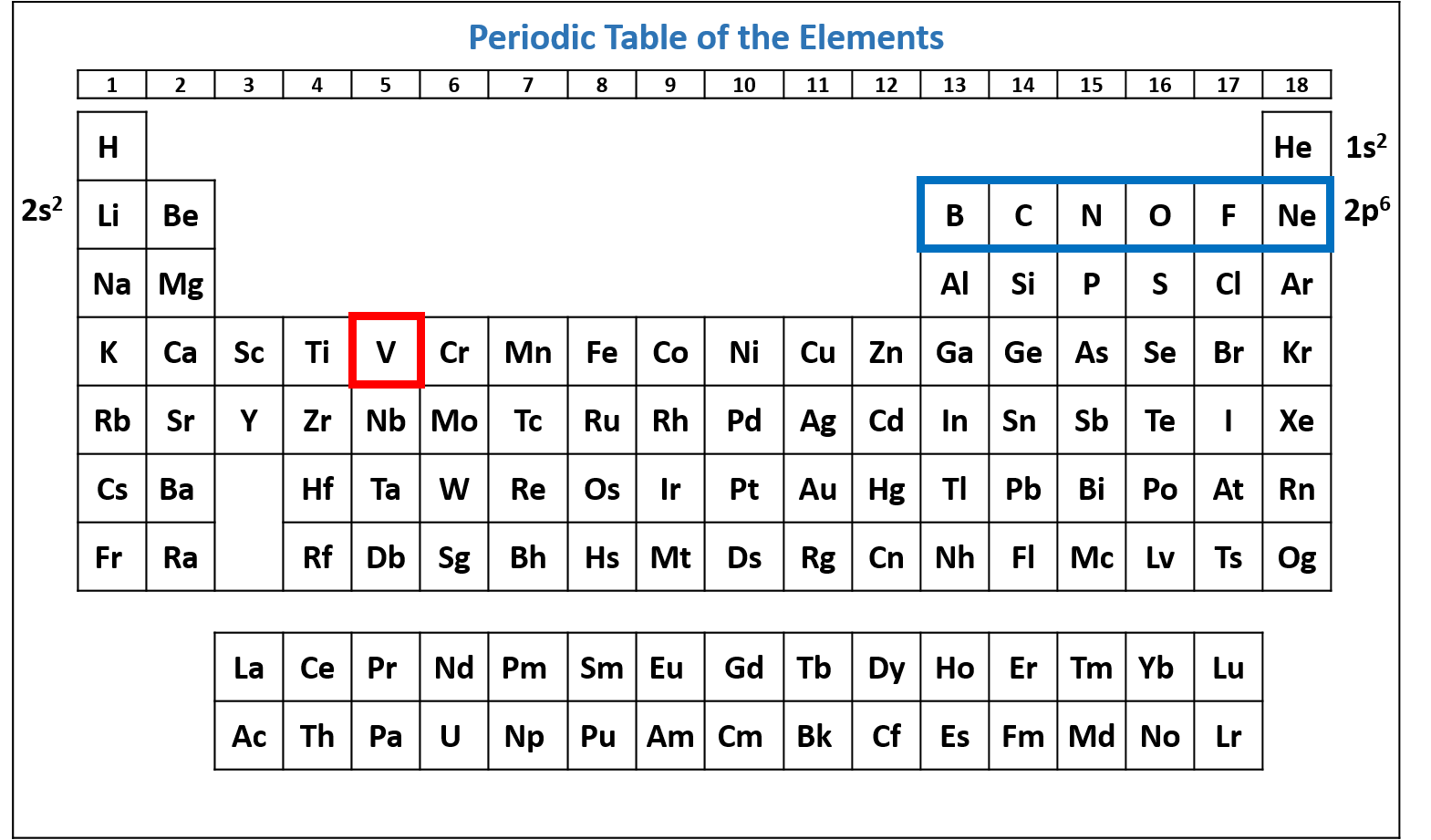
Figure \(\PageIndex{4}\): Periodic table of the elements with the 2p6 subshell highlighted. V has six electrons in the 2p subshell. (CC-BY-NC-SA; Kathryn A. Newton) 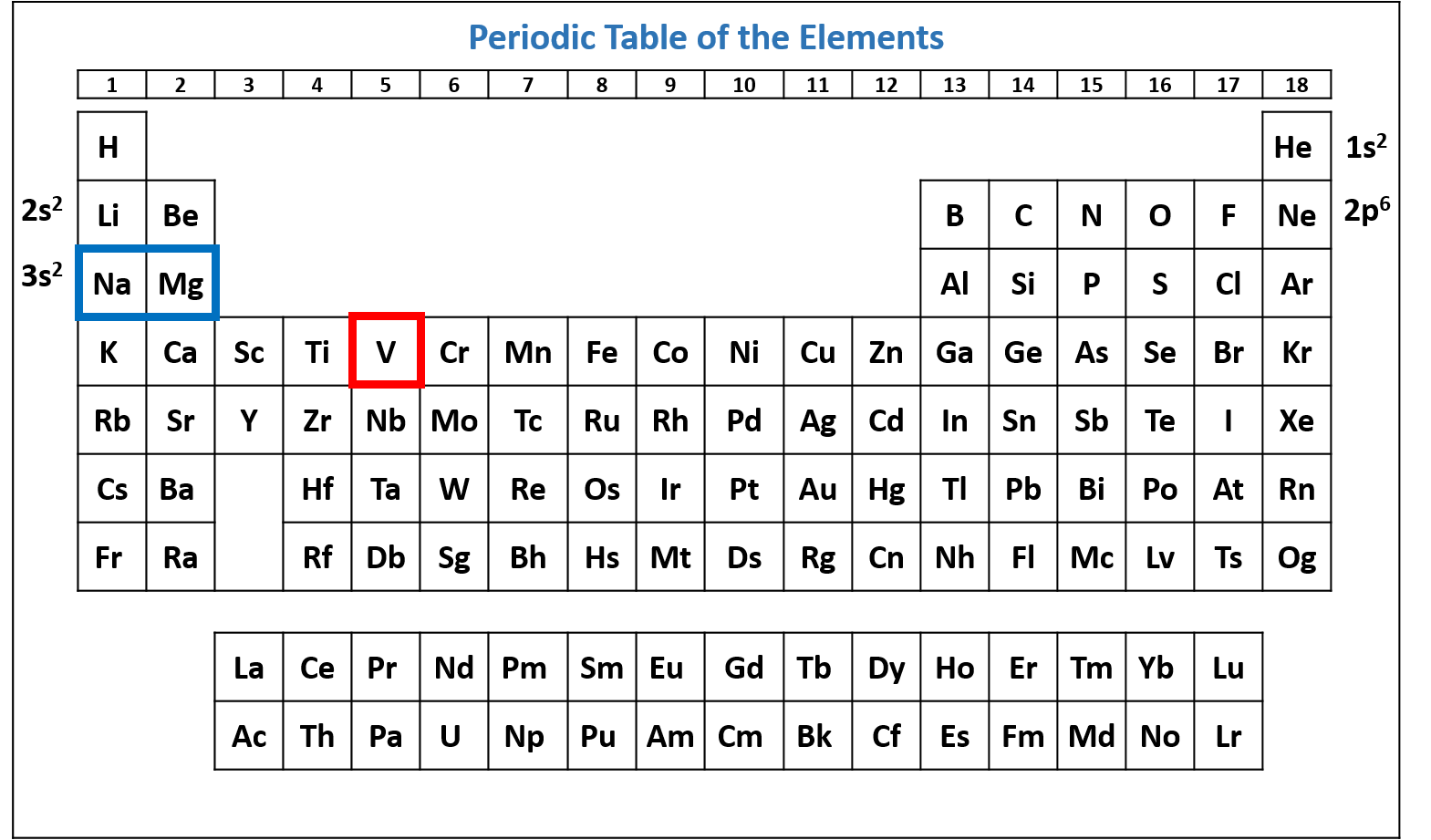
Figure \(\PageIndex{5}\): Periodic table of the elements with the 3s2 subshell highlighted. V has two electrons in the 3s subshell. (CC-BY-NC-SA; Kathryn A. Newton) 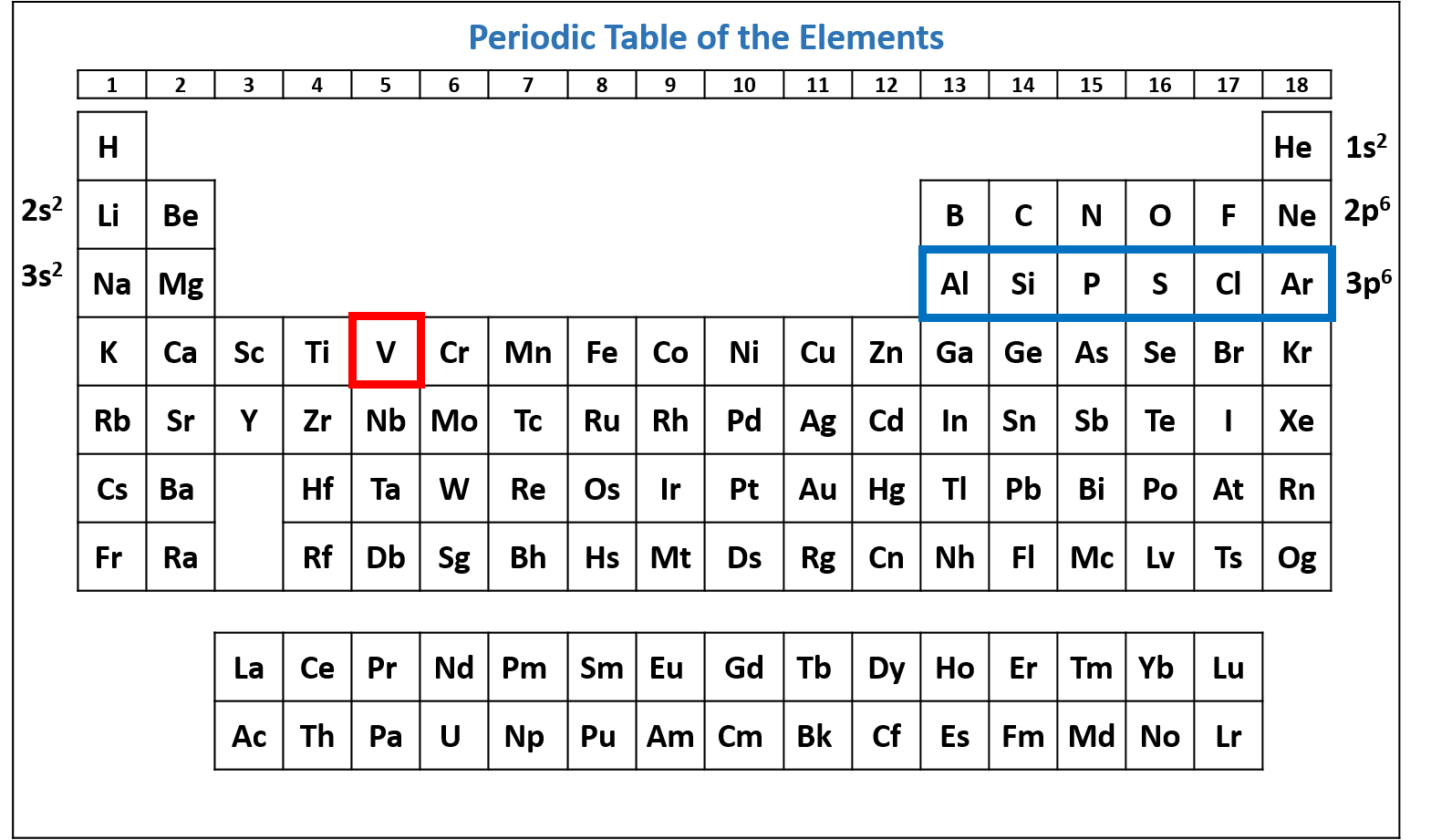
Figure \(\PageIndex{6}\): Periodic table of the elements with the 3p6 subshell highlighted. V has six electrons in the 3p subshell. (CC-BY-NC-SA; Kathryn A. Newton) 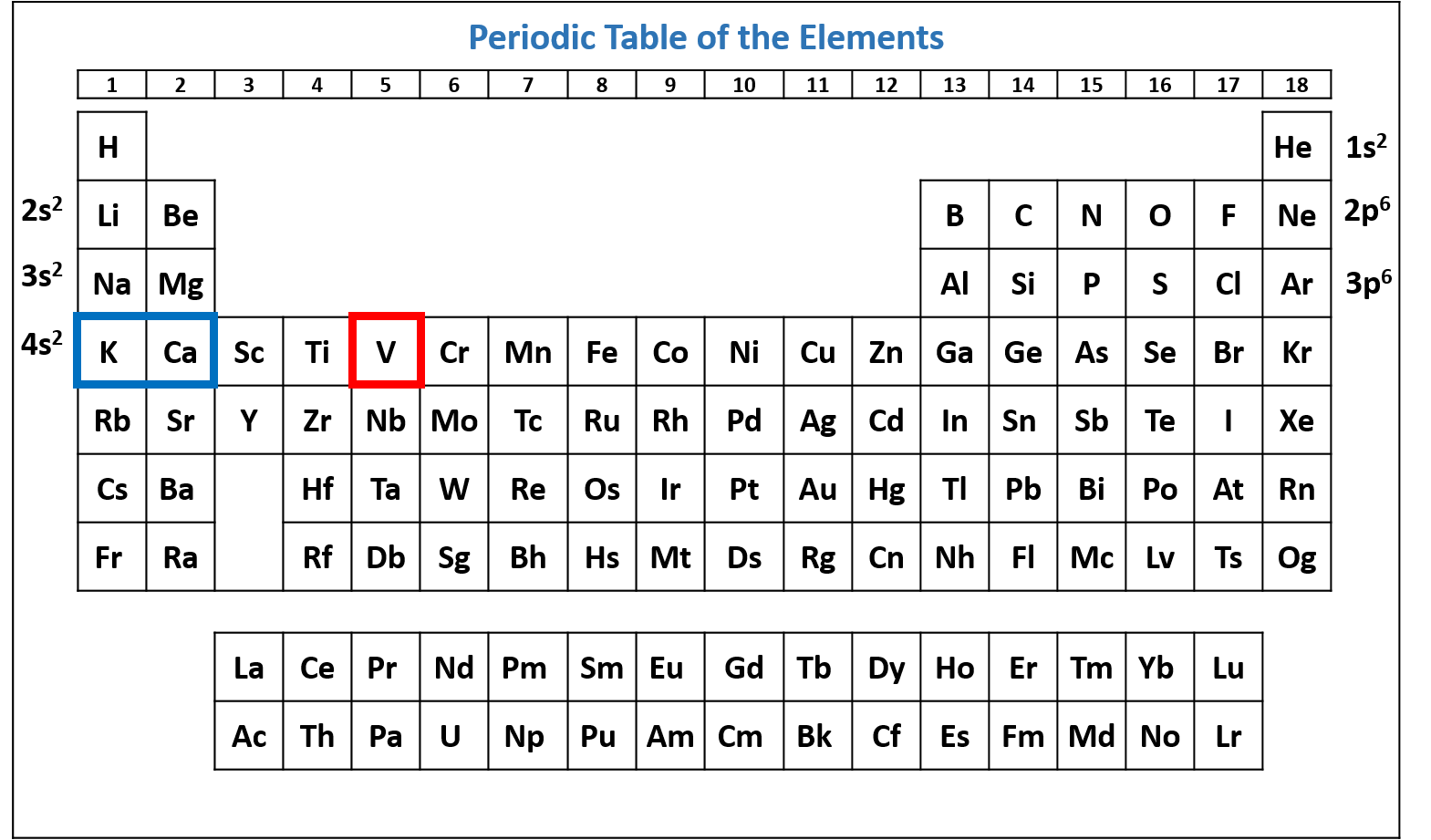
Figure \(\PageIndex{7}\): Periodic table of the elements with the 4s2 subshell highlighted. V has two electrons in the 4s subshell. (CC-BY-NC-SA; Kathryn A. Newton) 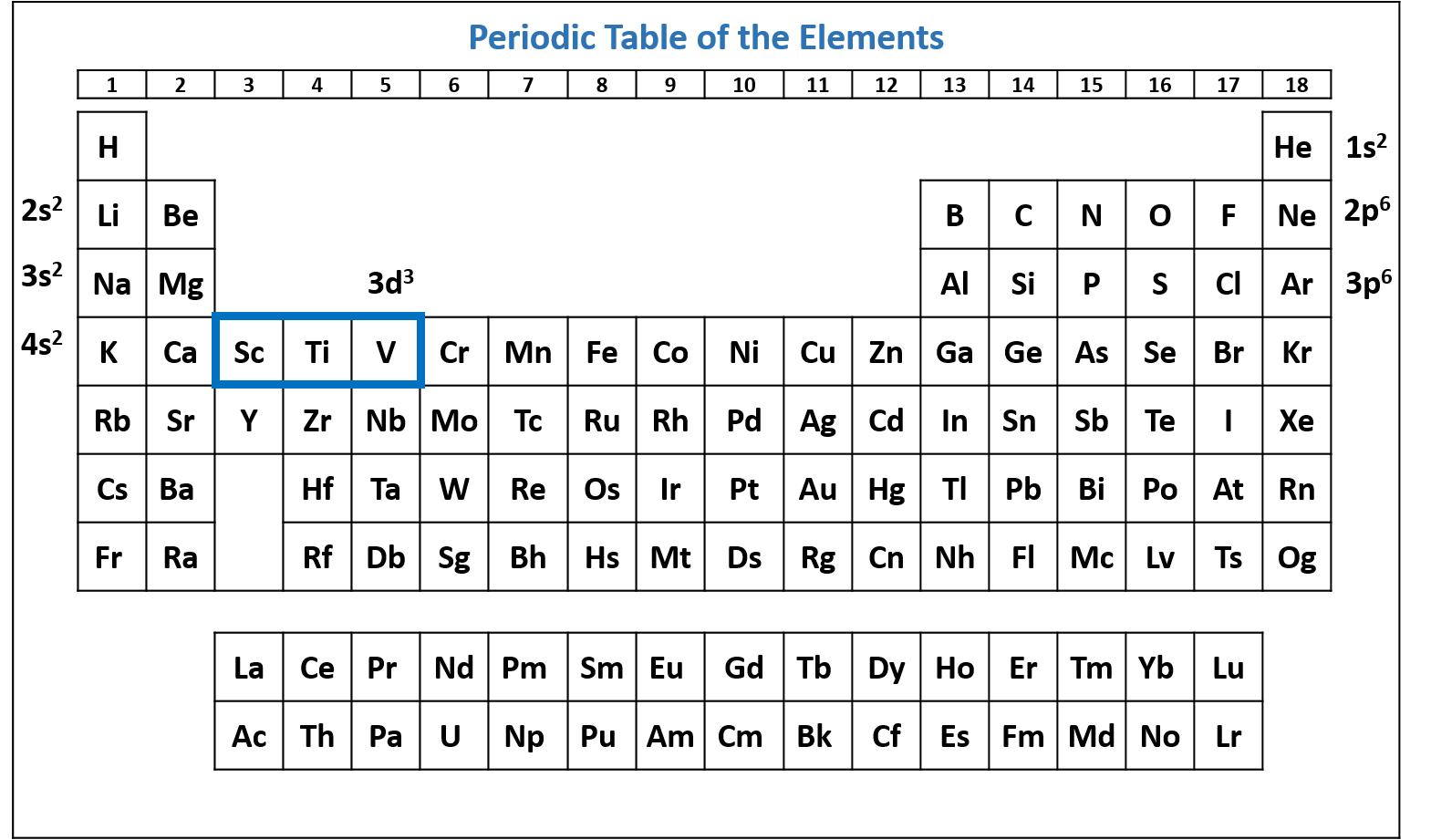
Figure \(\PageIndex{8}\): Periodic table of the elements with the 3d3 subshell highlighted. V has three electrons in the 3d subshell. (CC-BY-NC-SA; Kathryn A. Newton) - Combine the list into a single electron configuration. V's electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d3 or [Ar] 4s2 3d3
Write the ground state electron configuration for osmium (Os).
Solution
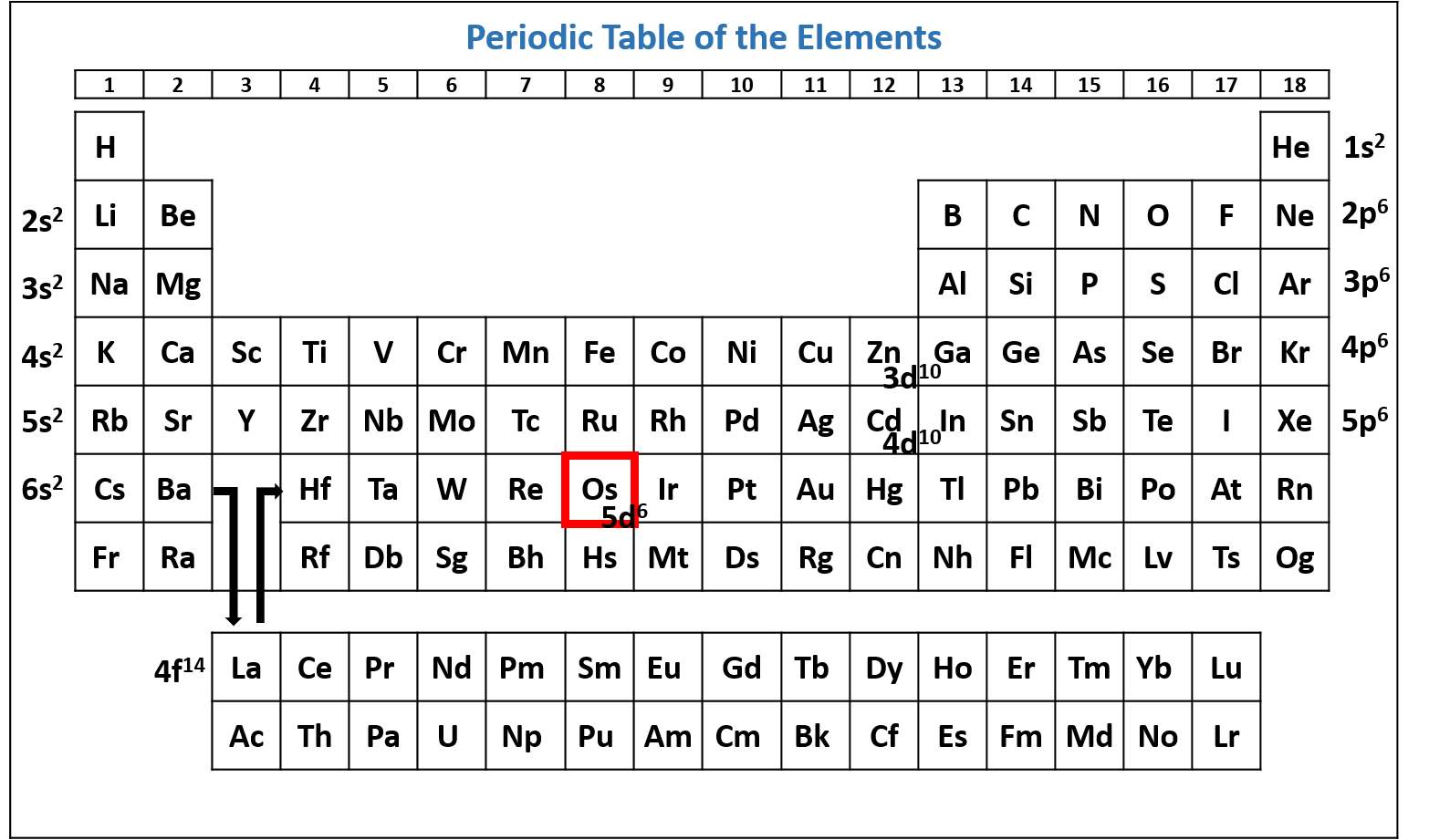
Exceptions to Expected Electron Configurations
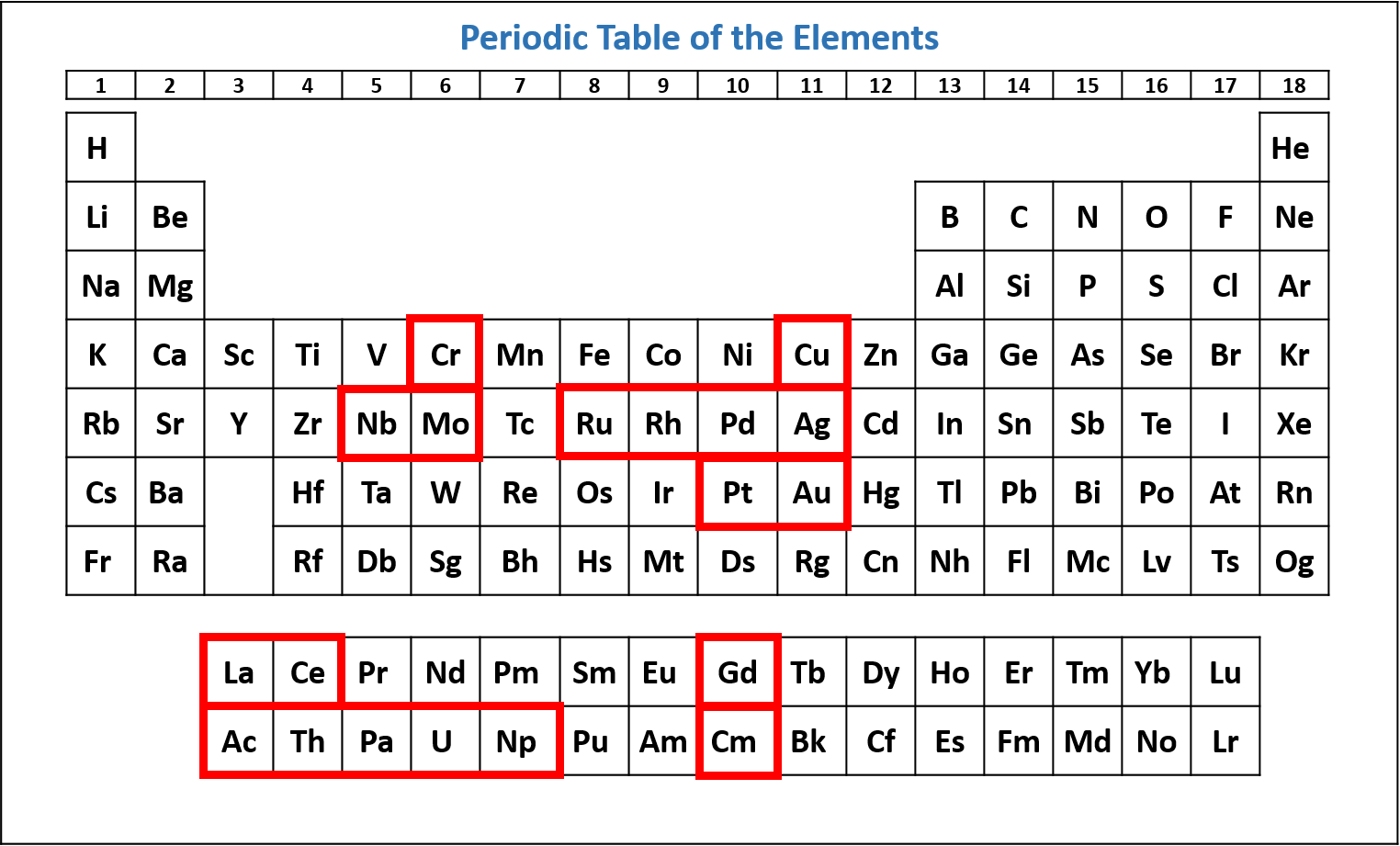
There are some exceptions to the order of filling of orbitals that is shown in Figure 1.6.1. For example, the electron configurations of the transition metals chromium (Cr) and copper (Cu), are not those we would expect. Rather, Cr and Cu take on half-filled and fully-filled 3d configurations.
The electron configuration of chromium (Cr) includes a half-filled 3d subshell.
Cr: 1s2 2s2 2p6 3s2 3p6 4s1 3d5
The electron configuration of copper (Cu) includes a fully-filled 3d subshell.
Cu: 1s2 2s2 2p6 3s2 3p6 4s1 3d10
The actual electron configuration of these elements may be rationalized in terms of an added stability associated with a half-filled (ns1, np3, nd5, nf7) or filled (ns2, np6, nd10, nf14) subshell. In fact, this "special stability" is really another consequence of the instability caused by pairing an electron with another in the same orbital. (There are repulsive forces between two electrons in the same orbital.) Recall the small differences in energy between higher energy subshells. In the case of Cr, the repulsive forces between two electrons in the 4s would be greater than the energy gained by shifting an electron into the 3d. Thus, the added stability is enough to shift an electron from one orbital to another. In the case of Cu, the repulsive forces between two electrons in the 4s orbital is greater than between two electrons paired in a 3d orbital so one electron is shifted from the 4s to the 3d. In heavier elements, other more complex effects can also be important and lead to many additional anomalies. For example, cerium has an electron configuration of [Xe]6s24f15d1, which is impossible to rationalize in simple terms. In most cases, however, these apparent anomalies do not have important chemical consequences. Figure \(\PageIndex{10}\) indicates which elements have a non-standard configuration.
Electron Configuration of Transition Metals: Electron Configuration of Transition Metals, YouTube(opens in new window) [youtu.be]
Electron Configurations of Transition Metal Ions
Transition metal elements are unique in the sense that they can have multiple oxidation states. While negative oxidation states are possible for some transition metal elements, we will focus on the positive oxidation states, which are more common.
Writing an electron configuration for a transition metal ion starts with the same steps as writing the configuration of an s- or p-block cation: Write the electron configuration for the neutral atom and then determine the number of electrons that are lost to form the cation. The electrons will be removed from the highest energy shell in the neutral configuration. This means that electrons will be removed from the 4s subshell before any electrons are removed from the 3d subshell, and similarly electrons will be removed from the 5s subshell before they are removed from the 4d subshell. If the highest energy s subshell is empty, then additional electrons will be removed from the highest energy d subshell.
Write an electron configuration for V4+.
Solution
- Write the configuration of the neutral atom. V: 1s2 2s2 2p6 3s2 3p6 4s2 3d3
- Determine how many electrons were lost. V4+ was formed by the loss of four electrons.
- Remove electrons from the highest shell, starting with the highest energy subshell.
V+: 1s2 2s2 2p6 3s2 3p6 4s1 3d3
V2+: 1s2 2s2 2p6 3s2 3p6 4s0 3d3
V3+: 1s2 2s2 2p6 3s2 3p6 4s0 3d2
V4+: 1s2 2s2 2p6 3s2 3p6 4s0 3d1, which is typically written as 1s2 2s2 2p6 3s2 3p6 3d1 or [Ar] 3d1
Problems
Write the expanded and shortened ground state electron configuration for nickel (Ni).
- Answer
-
Expanded: 1s2 2s2 2p6 3s2 3p6 4s2 3d8
Noble Gas: [Ar] 4s2 3d8
Write the expanded and shortened ground state electron configuration for rhodium (Rh).
- Answer
-
Expanded: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d7
Noble Gas: [Kr] 5s2 4d7
Write the ground state electron configuration for Ni2+.
- Answer
-
Neutral Ni: 1s2 2s2 2p6 3s2 3p6 4s2 3d8
Ni2+: 1s2 2s2 2p6 3s2 3p6 3d8 or [Ar] 3d8
Write the ground state electron configuration for Rh3+.
- Answer
-
Neutral Rh: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d7
Rh3+: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 4d6 or [Kr] 4d6
Osmium is stable with oxidation states of +2, +3, +4, and +8. Write the electron configuration for Os2+ and Os3+.
- Answer
-
Neutral Os: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d6
Os2+: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s0 4f14 5d6 or [Xe] 4f14 5d6
- Answer
-
Os3+: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s0 4f14 5d5 or [Xe] 4f14 5d5
References
- Petrucci, Ralph. Harwood, William. Herring, Geoffrey. Madura, Jeffery. General Chemistry: Principles and Modern Applications, 9th Edition. Pearson Education, Inc. New Jersey, 2007.
- Hein, et al. Introduction to General, Organic, and Biochemistry. 9th ed. Hoboken, NJ 2009.
Contributors
- Liza Chu (UCD)

