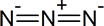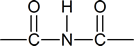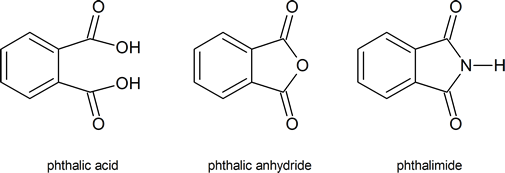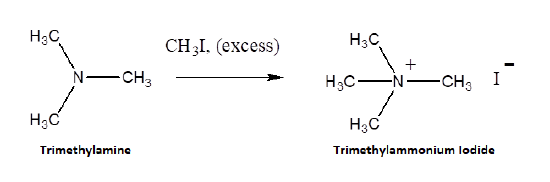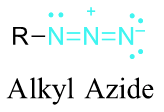Objectives
After completing this section, you should be able to
- write equations to illustrate the synthesis of amines by
- reduction of nitriles or amides and nitro compounds.
- reactions involving alkyl groups:
- SN2 reactions of alkyl halides, ammonia and other amines.
- nucleophilic attack by an azide ion on an alkyl halide, followed by reduction of the azide so formed.
- alkylation of potassium phthalimide, followed by hydrolysis of the N‑alkyl phthalimide so formed (i.e., the Gabriel synthesis).
- reductive amination of aldehydes or ketones.
- Hofmann or Curtius rearrangements.
- write detailed mechanisms for each of the steps involved in the synthetic routes outlined in Objective 1.
- identify the product or products formed when
- a given nitrile or amide is reduced using lithium aluminum hydride.
- a given alkyl halide is reacted with ammonia or an alkylamine.
- a given alkyl halide is reacted with azide ion and the resulting product is reduced.
- a given alkyl halide is reacted with potassium phthalimide and the resulting product is hydrolyzed.
- a given aldehyde or ketone is reacted with ammonia or an amine in the presence of nickel catalyst.
- a given amide is treated with halogen and base.
- a given acyl azide is heated and then hydrolyzed.
- identify the starting material, the other reagents, or both, needed to synthesize a given amine by any of the routes listed in Objective 1.
- write a general equation to illustrate the preparation of an arylamine by the reduction of a nitro compound, and balance such an equation.
- identify the product formed from the reduction of a given aromatic nitro compound.
- identify the organic compound, the inorganic reagents, or both, needed to prepare a given arylamine.
Key Terms
Make certain that you can define, and use in context, the key terms below.
- azide synthesis
- Curtius rearrangement
- Hofmann rearrangement
- Gabriel synthesis
- imide
- reductive amination
Study Notes
You may wish to review the mechanism of SN2 reactions which is discussed in some detail in Sections 11.2 and 11.3.
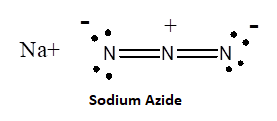
Azide synthesis is the first method on the table of synthesis of primary amines. The Lewis structure of the azide ion, N3−, is as shown below.
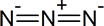
An “imide” is a compound in which an N$\ce{-}$H group is attached to two carbonyl groups; that is,
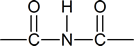
You should note the commonly used trivial names of the following compounds.
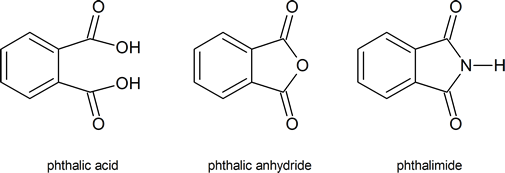
The phthalimide alkylation mentioned in the reading is also known as the Gabriel synthesis.
If necessary, review the reduction of nitriles (Section 20.7) and the reduction of amides (Section 21.7).
Before you read the section on reductive amination you may wish to remind yourself of the structure of an imine (see Section 19.8).
The Hofmann rearrangement is usually called the Hofmann degradation. In a true rearrangement reaction, no atoms are lost or gained; however, in this particular reaction one atom of carbon and one atom of oxygen are lost from the amide starting material, thus the term “rearrangement” is not really appropriate. There is a rearrangement step in the overall degradation process, however: this is the step in which the alkyl group of the acyl nitrene migrates from carbon to nitrogen to produce an isocyanate.
Reduction of Nitriles, Amides, and Nitro Compounds
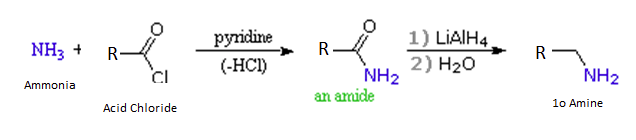
Acid chlorides react with ammonia to give amides, by an addition-elimination path, and these are reduced to amines by LiAlH4 (Section 21-7).
Alkyl halides can be converted to primary amines through a two-step process. First an SN2 reaction with a cyanide anion converts the alkyl halide into a nitrile. Then the nitrile is reduced to a primary amine by LiAlH4 (Section 20-7). During this reaction sequence an additional carbon atom is added.

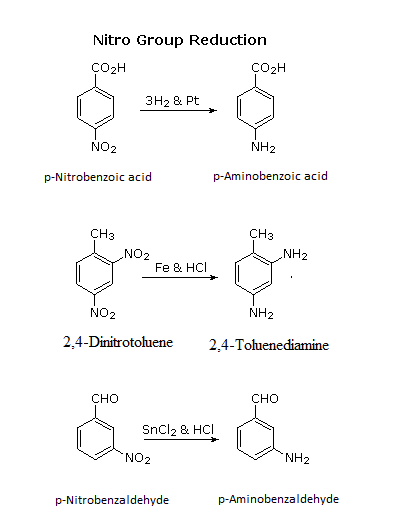
Arylamines are typically prepared by the reduction of a nitro group on an aromatic ring. Several methods for reducing nitro groups to amines are known. These include catalytic hydrogenation (H2 + platinum catalyst), and zinc, iron, or tin(II) chloride in dilute mineral acid. The procedures described above are sufficient for most cases. Catalytic hydrogenation can be problematic because it is known to reduce other functional groups such as alkenes, alkynes, and some carbonyl groups.
Exercise \(\PageIndex{1}\)
Propose structures of the starting materials needed to make the the following amines using the reduction of a nitrile or an amide:
a) CH3CH2CH2CH2NH2
b) (CH3CH2)2NH
c)
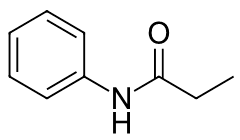
e) N-Propylaniline
- Answer
-
a) CH3CH2CH2CONH2
b) CH3CONHCH2CH3
c)
e)
SN2 Reactions of Alkyl Halides
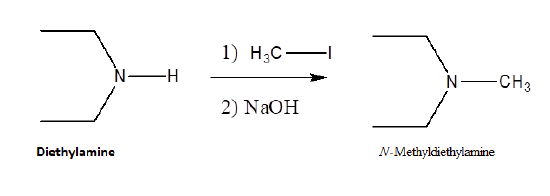
Because they posses lone pair electrons, ammonia and amines are considered good nucleophiles. Ammonia and amines can by alkylated by an SN2 reaction with alkyl halides. The product of the reaction is an ammonium salt where the negative counter-ion is the halogen of the alkyl halide. When the alkylated products are 1o, 2o, and 3o amines, they can be deprotonated with NaOH to produce the neutral amine. When a 3o amine is alkylated, a quaternary ammonium salt is produced.

Alkylation is an efficient method for the synthesis of 3o and 4o amines. However, when 1o and 2o amines are alkylated a mixture of products is typically produced. When ammonia is reacted with an alkylhalide an monoalkylammonium salt is formed.
RX + NH3 → RNH3+ + X-
It is possible for any remaining ammonia present in the reaction to deprotonate the ammonium salt to produce the neutral monoalkyl amine.
RNH3+ + NH3 → RNH2 + NH4+
The monoalkyl amine is then free to react with a second alkyl halide creating a dialkylammonium salt. This process can be repeated to to eventually to create a tetralkylammonium salt. Typically when a alkyl halide is reacted with ammonia, a mixture of mostly 1o and 2o amines, with trace amounts of 3o amines and 4o ammonium salts, is produced.
RNH2 + RX → R2NH2+ + X-
Example
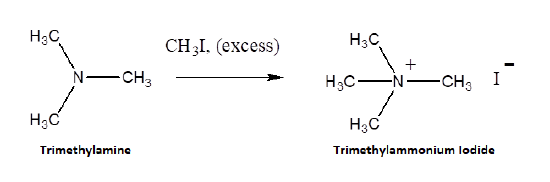
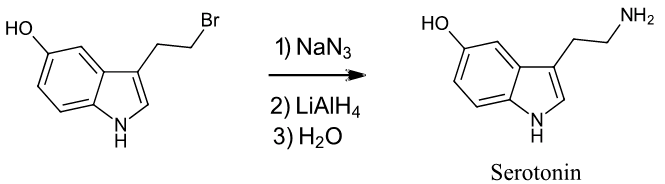
To synthesize a 1o or 2o amine, specific reactions are usually employed. A more efficient method starts with an SN2 reaction between a 1o or 2o alkyl halide and the nucleophilic azide anion (N3-) to produce an alkyl azide. The alkyl azide is not nucleophilic so it cannot react with additional alkyl halide to produce overalkylation. Alkyl azide is then reduced with LiAlH4 to produce a 1o amine.
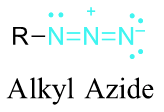
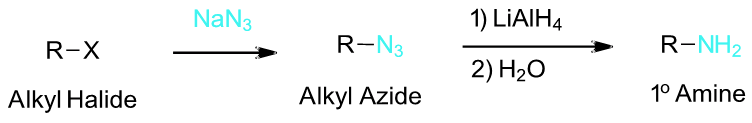
Example

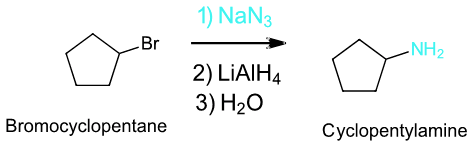
Gabriel Amine Synthesis
Another common method for the synthesis of 1o amines is called the Gabriel amine synthesis. This reaction starts with the deprotonation of phthalimide by a hydroxide base such as potassium hydroxide (KOH). The N-H hydrogen of an imide functional group is acidic because its conjugate base is resonance stabilized by two carbonyl groups. The phthalimide anion is nucleophilic and easily alkylated through an SN2 reaction with an alkyl halide. The resulting N-alkylated phthalimide then undergoes base hydrolysis to produce a 1o amine product. The mechanism for the base promoted hydrolysis of the N-alkylated phthalimide is analogous to the hydrolysis of an amide (Section 21-7).
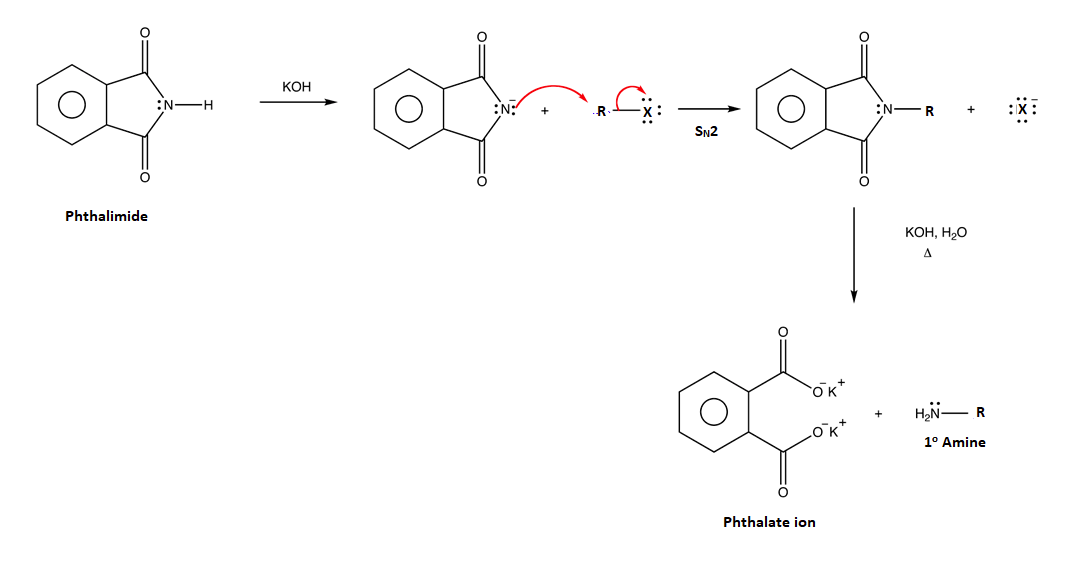
Example
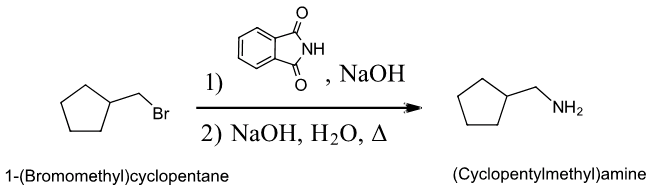
Exercises \(\PageIndex{1}\)
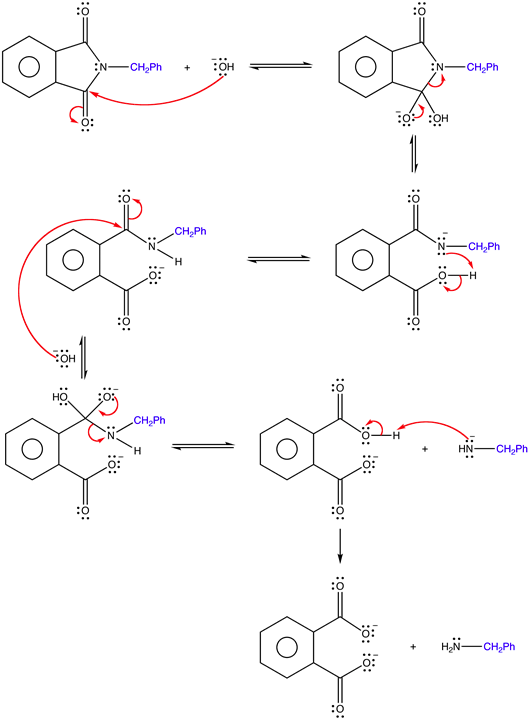
1) Write the mechanism for the base promoted hydrolysis of an N-alkyl phthalimide to create a 1o amine and the phthalate ion.
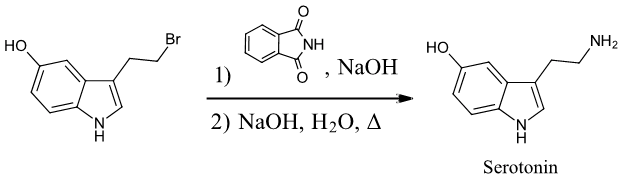
2) Starting with any alkyl halide, show two methods to synthesize the neurotransmitter serotonin.
- Answers
-
1)
2)
Reductive Amination
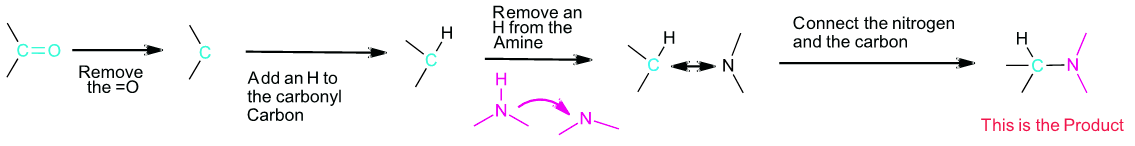
Aldehydes and ketones can be converted into 1o, 2o and 3o amines using reductive amination. The reaction takes place in two parts. The first step is the nucleophiic addition of ammonia, a 1o amine, or a 2o amine to a carbonyl group to form an imine (Section 19-8). The second step is the reduction of the imine to an amine using a hydride reducing agent. Some common reducing agents used for this reaction are: sodium borohydride (NaBH4), sodium cyanoborohydride (NaBH3CN), NaBH(OAc)3, or hydrogen gas (H2) over a nickel catalyst.
General Reaction

Mechanism
Reductive amination starts with the nucleophilic addition of ammonia or a 1o amine to an aldehyde or ketone forms a cyanohydrin intermediate. Subsequent dehydration forms an imine intermediate (Section 19-8). The imine undergoes hydride reduction in a similar fashion as C=O carbonyl bonds are reduced to an alcohol.

When a 2o amine is used for reductive amination, an enamine intermediate is formed which then undergoes hydride reduction to form a 3o amine.

Predicting the Products of a Reductive Amination
Examples


Biological Reductive Aminations
Reductive amination is used in the biosynthesis of the amino acid proline. The enzyme pyrroline-5-carboxylate synthase (P5CS) catalyzes glutamate undergoing an intramolecular imine formation to produce 1-pyrrolinium 5-carboxylate. Then the enzyme pyrroline-5-carboxylate reductase (PYCR) catalyzed the nucleophilic hydride reduction of the C=N bond to produce proline. The biological hydride reducing agent for this sequence is reduced nicotinamide adenine dinucleotide (NADH).

Planning a Synthesis using a Reductive Amination
If you are trying to develop a synthesis of an amine molecule, the key bond break is a C-N bond in the target molecule. There may be multiple C-N bonds present, so each should be broken separately to produce a possible set of starting materials. Because the amine is commonly used in excess during a reductive animation, the pathway which provides the simplest amine starting material is preferred. The carbon from the broken C-N bond goes on to become a carbonyl starting material. The nitrogen gains a hydrogen to become the amine starting material.
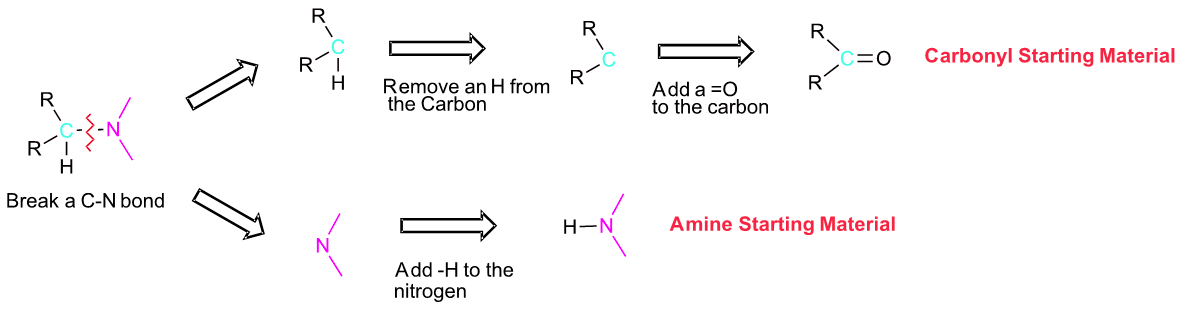
Worked Exercise \(\PageIndex{1}\)
Show how N-methylbenzylamine can be synthesized using a reductive amination. If more than one pathway is possible, draw them both and determine which one would be preferred.

- Answer
-
Pathway 1
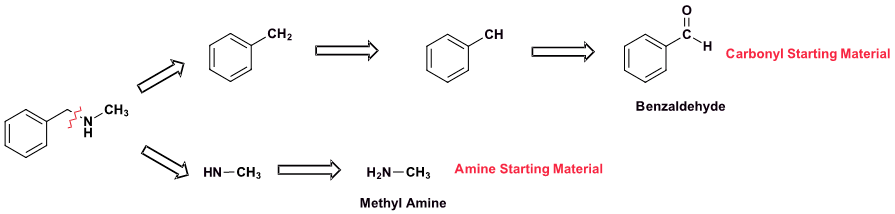
Solution 1

Pathway 2
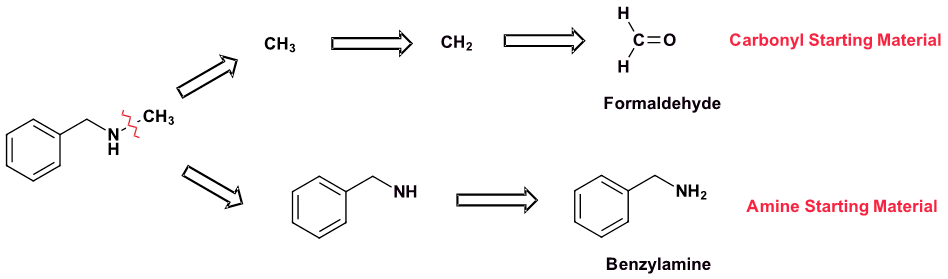
Solution 2

Because N-methylbenzylamine has two C-N bonds there are two possible synthesis pathways. Pathway 1 is most likely preferred because it uses the simplest amine starting material, methyl amine.
Exercise \(\PageIndex{1}\)
Provide starting materials which could be used to prepare the following compounds using a reductive amination. Show all possible pathways.
1)
a)

b)

c)

2) Please give the starting materials which could provide the following molecule using a reductive amination.
- Answers
- 1)
-
a)

b)

c)

2)

Hofmann Rearrangement
The Hofmann rearrangement occurs when a 1o amide is reacted with bromine (Br2) and a base. The products are a 1o amine with one less carbon and carbon dioxide (CO2).
General Reaction
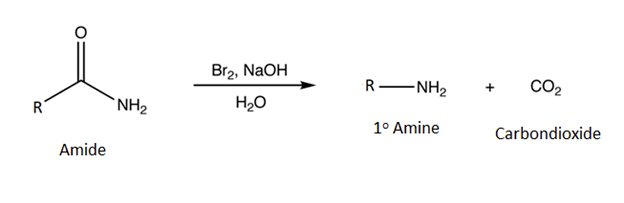
Example

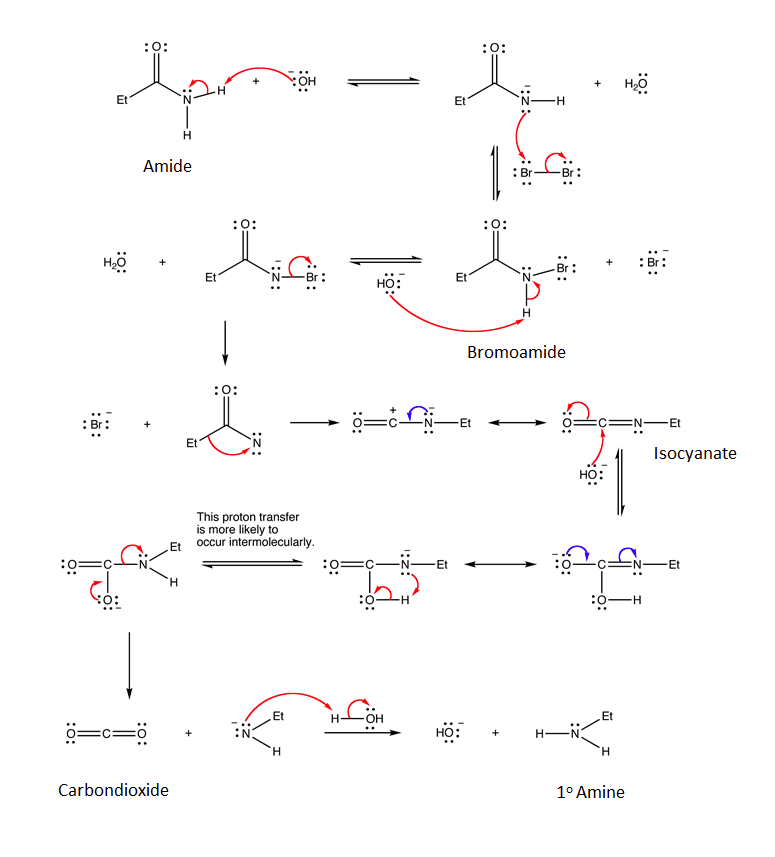
Mechanism
The mechanism for the Hofmann rearrangement is quite complex. The mechanism starts with the deprontation of the primary amide by a base. The nitrogen is brominated during an SN2 reaction with Br2 to produce an N-bromoamide. Bromide is eliminated as a leaving group to produce a electron deficient nitrene-like nitrogen. Electrons from the adjacent C-C bond migrate to the nitrogen as part of a rearrangment to finally produce an isocyanate. The carbon in the isocyante is electrophilic and reacts with a nucleophilic hydroxide to create a C-O bond. After movement of electrons through resonance and a proton transfer a carbamate intermediate is formed. The carbamate decomposes to create an amide anion and a stable CO2 molecule. Finally, water protonates the amide anion to produce a 1o amine.
Curtius Rearrangement
The Curtius Rearrangement is another method used to synthesize a 1o amine.
General Reaction
The Curtius rearrangement involves an acyl azide reacting with water and heat to produce a 1o amine along with CO2 and N2. The acyl azide is usually made by a nucleophilic substitution of an acid chloride with sodium azide (NaN3)
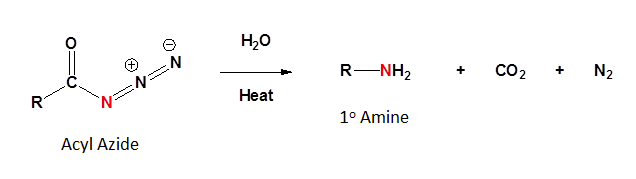
Mechanism
The mechanism of the Curtius rearrangement is quite similar to the Hoffman rearrangement. The loss of N2 as a leaving group creates an electron deficient nitrogen. The adjacent -R group migrates to form an isocyanate. Reaction of the isocyanate forms a carbamate which decomposes to form a 1o amine and CO2.

Example

Planning a Synthesis using a Hoffman or Curtius Rearrangement
Because the amide and the acyl azide required for these rearrangements both are best made from an acid chloride, synthesis of amines using these reaction are best started from the corresponding carboxylic acid. For retrosynthetic analysis starting from the desired amine product - the key break is the C-NH2 bond. The amine is removed and replaced with a -CH2CO2H fragment to provide the required carboxylic acid starting material.

Worked Example \(\PageIndex{1}\)
Show how the following molecule could be prepared from a carboxylic acid starting material using both the Hoffman and Curtius rearrangements.

- Answer
-
First determine the structure of the carboxylic acid starting material. This will be the starting material for both rearrangements. In the first step, convert the carboxylic acid into an acid halide using thionyl chloride (SOCl2). Then use the required conditions for each rearrangement.

Solution
The top solution shows the Curtius Rearrangement, while the bottom is an example using the Hofmann Rearrangement.

Exercises \(\PageIndex{1}\)
1) Using a carboxylic acid starting material explain how to prepare the following molecules using both the Hoffman and Curtius rearrangements.
a)

b)

- Answers
-
1)
a) The top solution shows the Curtius Rearrangement, while the bottom is an example using the Hofmann Rearrangement.

b) The top solution shows the Curtius Rearrangement, while the bottom is an example using the Hofmann Rearrangement.