5.8: Ligand Exchange Reactions (Thermodynamics)
- Page ID
- 300449
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Replacing aqua ligands with ammine ligands
If you add ammonia solution to a solution containing hexaaquacopper(II) ions, [Cu(H2O)6]2+, four of the water molecules are eventually replaced by ammonia molecules to give [Cu(NH3)4(H2O)2]2+. This can be written as an equilibrium reaction to show the overall effect:
\[\ce{[Cu(H2O)6]^{2+} + 4NH3 <=> [Cu(NH3)4(H2O)2]^{2+} + 4H2O} \label{all}\]
In fact, the water molecules get replaced one at a time, and so this is made up of a series of part-reactions:
\[\ce{[Cu(H2O)6]^{2+} + NH3 <=> [Cu(NH3)(H2O)5]^{2+} + H2O}\label{step1}\]
\[\ce{ [Cu(NH3)(H2O)5]^{2+} + NH3 <=> [Cu(NH3)2(H2O)4]^{2+} + H2O} \label{step2}\]
\[\ce{[Cu(NH3)2(H2O)4]^{2+} + NH3 <=> [Cu(NH3)3(H2O)3]^{2+} + H2O} \label{step3}\]
\[\ce{[Cu(NH3)3(H2O)3]^{2+} + NH3 <=> [Cu(NH3)4(H2O)2]^{2+} + H2O} \label{step4}\]
Although this can look a bit daunting at first sight, all that is happening is that first you have one, then two, then three, then four water molecules in total replaced by four ammine ligands.
Individual stability constants
Let's take a closer look at the first of these equilibria (Equation \ref{step1}). Like any other equilibrium, this one has an equilibrium constant, \(K_c\) - except that in this case, we call it a stability constant. Because this is the first water molecule to be replaced, we call it \(K_1\) and is given by this expression:
\[K_1 = \dfrac{[\ce{[Cu(NH3)(H2O)5]^{2+}}]}{\ce{[[Cu(H2O)6]^{2+}}][\ce{NH3]}}\]
There are two points of possible confusion here - one minor, one more important!
- First, the square brackets have changed their meaning! Square brackets are often used to keep everything in a complex ion together and tidy. Here, they have reverted to their other use, which implies concentrations in mol dm-3.
- In order to avoid complete confusion, the square brackets keeping the complexes together have been removed entirely.
- More importantly, if you compare the equilibrium constant expression with the equation, you will see that the water on the right-hand side hasn't been included. That is normal practice with these expressions.
- Because everything is dissolved in water, the water is present as a huge excess. Generating a little bit more during the reaction is going to make no effective difference to the total concentration of the water in terms of moles of water per dm3.
- The concentration of the water is approximately constant. The equilibrium constant is defined so that you avoid having an extra unnecessary constant in the expression.
With that out of the way, let's go back to where we were - but introduce a value for K1:
![]()

The value of the equilibrium constant is fairly large, suggesting that there is a strong tendency to form the ion containing an ammonia molecule. A high value of a stability constant shows that the ion is easily formed. Each of the other equilibria above also has its own stability constant, K2, K3 and K4. For example, K2 is given by:
![]()

The ion with two ammonias is even more stable than the ion with one ammonia. You could keep plugging away at this and come up with the following table of stability constants:
| ion | Kn | value (mol-1 dm3) | log Kn |
|---|---|---|---|
| [Cu(NH3)(H2O)5]2+ | K1 | 1.78 x 104 | 4.25 |
| [Cu(NH3)2(H2O)4]2+ | K2 | 4.07 x 103 | 3.61 |
| [Cu(NH3)3(H2O)3]2+ | K3 | 9.55 x 102 | 2.98 |
| [Cu(NH3)4(H2O)2]2+ | K4 | 1.74 x 102 | 2.24 |
You will often find these values quoted as log K1 or whatever. All this does is tidy the numbers up so that you can see the patterns more easily. The ions keep on getting more stable as you replace up to 4 water molecules, but notice that the equilibrium constants are gradually getting smaller as you replace more and more water ligands. This is common with individual stability constants.
Overall Formation constants
The overall stability constant is simply the equilibrium constant for the total reaction:
![]()
It is given by this expression:

You can see that overall this is a very large equilibrium constant, implying a high tendency for the ammonia ligands to replace the water ligands. The "log" value is 13.1. This overall value is found by multiplying together all the individual values of K1, K2 and so on. To find out why that works, write down expressions for all the individual values (the first two are done for you above), and then multiply those expressions together. You will find that all the terms for the intermediate ions cancel out to leave you with the expression for the overall stability constant.
Summary
Whether you are looking at the replacement of individual water molecules or an overall reaction producing the final complex ion, a stability constant is simply the equilibrium constant for the reaction you are looking at. The larger the value of the stability constant, the further the reaction lies to the right. That implies that complex ions with large stability constants are more stable than ones with smaller ones.
Stability constants and entropy - the chelate effect
The chelate effect is an effect which happens when you replace water (or other simple ligands) around the central metal ion by multidentate ligands like 1,2-diaminoethane (en) or EDTA. Compare what happens if you replace two water ligands around a [Cu(H2O)6]2+ ion with either 2 ammonia ligands or one en ligand.
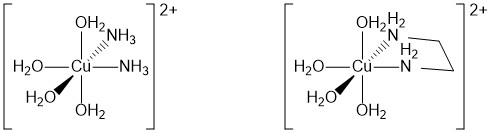
Chelates are much more stable than complex ions formed from simple monodentate ligands. The overall stability constants for the two ions are:
| ion | log K |
|---|---|
| [Cu(NH3)2(H2O)4]2+ | 7.86 |
| [Cu(H2O)4(en)]2+ | 10.6 |
The reaction with the 1,2-diaminoethane could eventually go on to produce a complex ion [Cu(en)3]2+. The overall stability constant for this (as log K) is 18.7. Another copper-based chelate comes from the reaction with EDTA. This also has a high stability constant - log K is 18.8. However many examples you take, you always find that a chelated complex is more stable than one with only monodentate ligands. This is known as the chelate effect.
The reason for the chelate effect
If you compare the two equilibria below, the one with the en has the higher equilibrium (stability) constant (for values, see above).
![]()
![]()
The enthalpy changes of the two reactions are fairly similar. You might expect this because in each case you are breaking two bonds between copper and oxygen atoms and replacing them by two bonds between copper and nitrogen atoms. However, when considering the reverse reaction there is a bit of a difference in enthalpy. If one Cu-N bond breaks, the NH3 ligand can be replaced by a water ligand. However, for en to leave, both Cu-N bonds to the ligand must be broken. If only one Cu-N bond to en is broken, the unbound nitrogen atom remains in close proximity to the copper atom, making it very likely that it will just reform the Cu-N bond.
Another factor in the chelate effect is the difference has to do with the entropy change during each reaction.
Entropy is most easily thought of as a measure of disorder. Any change which increases the amount of disorder increases the tendency of a reaction to happen.
If you look again at the two equiilbria, you might notice that the en equilibrium does lead to an increase in the entropy. There are only two species on the left-hand side of the equation, but three on the right.
![]()
You can obviously get more entropy out of three species than out of only two. Compare that with the other equilibrium. In this case, there is no change in the total number of species before and after reaction, and so no useful contribution to an increase in entropy.
![]()
In the case of the complex with EDTA, the increase in entropy is very pronounced.
![]()
Here, we are increasing the number of species present from two on the left-hand side to seven on the right. You can get a major amount of increase in disorder by making this change.
Reversing this last change is going to be far more difficult in entropy terms. You would have to move from a highly disordered state to a much more ordered one. That isn't so likely to happen, and so the copper-EDTA complex is very stable.
Summary
Complexes involving multidentate ligands are more stable than those with only monodentate ligands. The underlying reason for this is that each multidentate ligand displaces more than one water molecule. This leads to an increase in the number of species present in the system, and therefore an increase in entropy. An increase in entropy makes the formation of the chelated complex more favorable.
Contributors and Attributions
Jim Clark (Chemguide.co.uk)


