5.2: Ligands and Nomenclature
- Page ID
- 296130
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Ligand Denticity
Ligands can also be classified based on whether they bind to the metal center through a single site on the ligand or whether they bind at multiple sites. Ligands which bind through only a single site are called monodentate at the Latin word for tooth; in contrast those those which bind through multiple sites are called chelating after the Greek \(\chi \alpha \lambda \epsilon \) for “claw”. These relationships are summarized in Figure \(\sf{\PageIndex{1}}\).
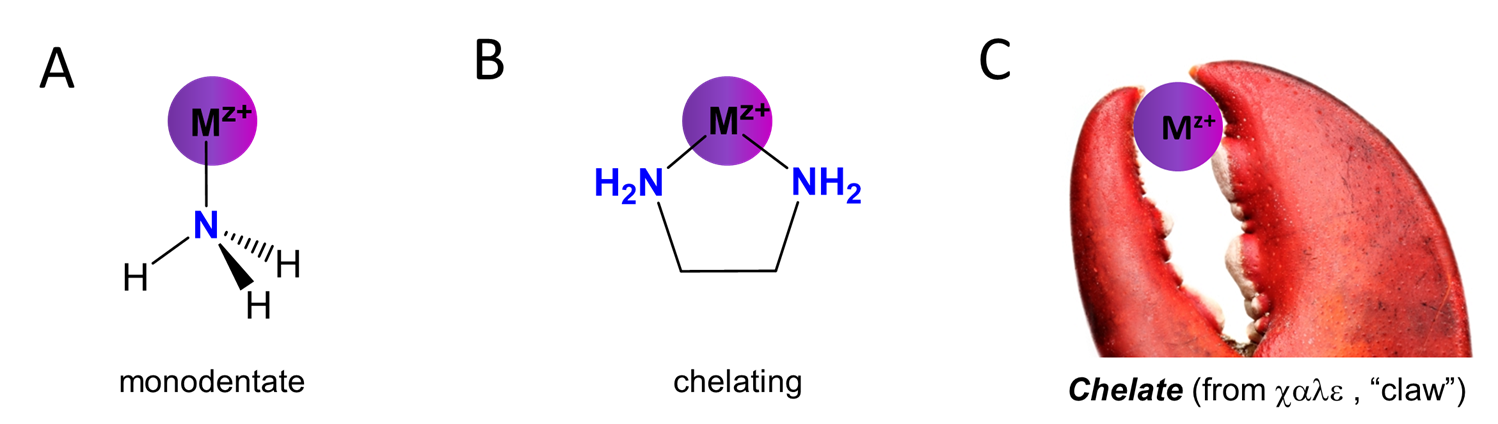
Figure \(\sf{\PageIndex{1}}\). (A) Ammonia is monodentate ligand while (B) ethylene diamine is a chelating ligand owing to its capacity to bind metals via its two amine functional groups. (C) Chelating ligands act like a Lobster claw in attaching to the metal via multiple sites. The lobster claw image is adapted from https://www.clipart.email/download/1127636.html. Otherwise this work by Stephen Contakes is licensed under a Creative Commons Attribution 4.0 International License.
Following naturally from the classification of non-chelating ligands as monodentate, chelating ligands are further classified according to the number of sites which they can use to bind a metal center. This number of binding sites is called the denticity and ligands are referred to as monodentate (non chelating), bidentate, tridentate, etc. based on the number of sites available. Ligands with two binding sites have a denticity of two and are said to be bidentate; those with three are tridentate, four tetradentate, and so on. To illustrate this classification system examples of chelating ligands classified according to denticity are given in Figure \(\sf{\PageIndex{2}}\).
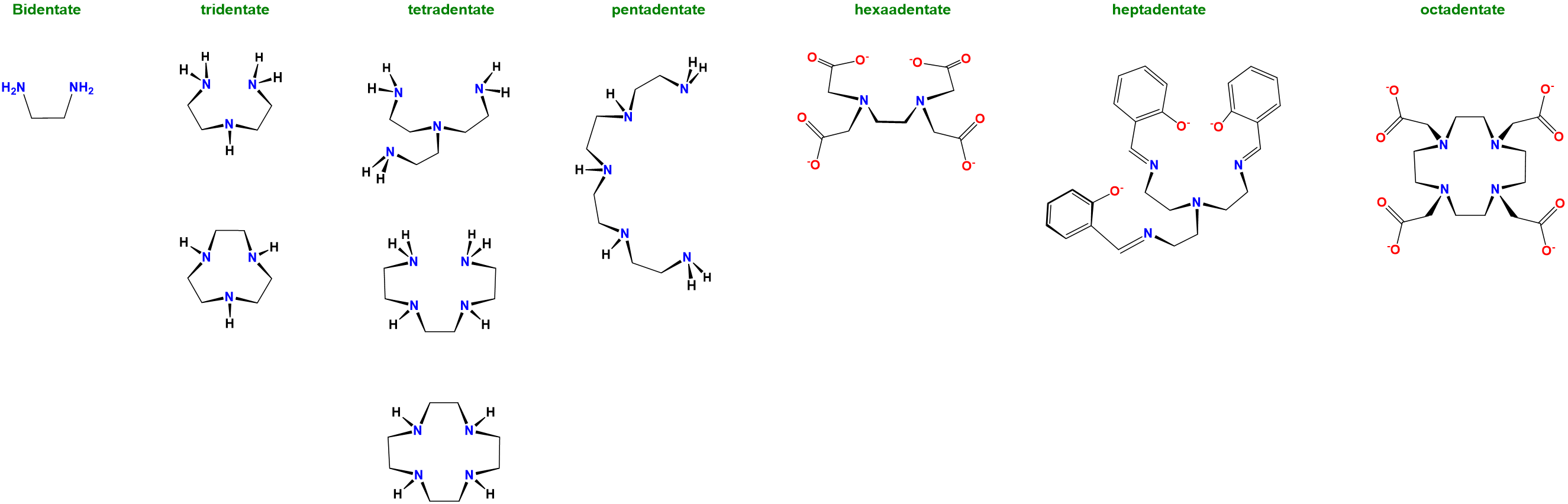
Figure \(\sf{\PageIndex{2}}\). A selection of chelating ligands classified according to denticity. this work by Stephen Contakes is licensed under a Creative Commons Attribution 4.0 International License.
The classification of several of the ligands in Figure \(\sf{\PageIndex{2}}\) requires a bit of explanation, specifically as to why the denticity of the carboxylate-containing ligands is less than the total number of lone-pair bearing oxygen and nitrogen atoms present. This is because only one of the carboxylate oxygen atoms is counted. Only one is counted because in most cases only one oxygen binds to a metal at any given time. When that oxygen is bound the other oxygen faces away from the metal, as depicted for the iron complex shown in Figure \(\sf{\PageIndex{3A}}\). Because only one oxygen per carboxylate typically binds only one is counted when assigning a ligand's denticity.
Although only one carboxylate oxygen usually binds to a metal it is still possible to bind a metal using both oxygen atoms. As shown in Figure \(\sf{\PageIndex{3B}}\) complexes in which both carboxylates bind to a metal are known, and in fact are common in the active sites of some enzymes. It is just that the binding of both oxygen atoms gives a strained four-membered ring that is usually unstable.
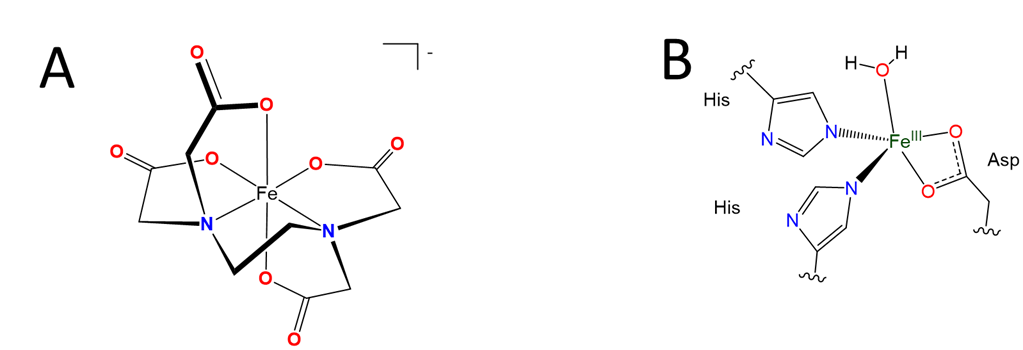
Figure \(\sf{\PageIndex{3}}\). (A) Only one oxygen per carboxylate counts towards the denticity of EDTA since on binding the other oxygen generally points away from the metal center, as in the structure of Fe(EDTA)-. This does not mean that both oxygens of a carboxylate can never both bind to metal centers in a complex. (B) Structures in which both oxygens of a carboxylate side chain bind to a metal are sometimes found in the active sites of some of the nonheme iron enzymes your body uses to break down amino acids. this work by Stephen Contakes is licensed under a Creative Commons Attribution 4.0 International License.
As was the case for carboxylate groups above, sometimes the classification of a group's denticity is based on experimental knowledge of their common binding modes. Carboxylates might commonly act as monodentate ligands but dithiocarbamates more commonly bind metals through both sulfur atoms (Figure \(\sf{\PageIndex{4}}\)) and are classified as bidentate.
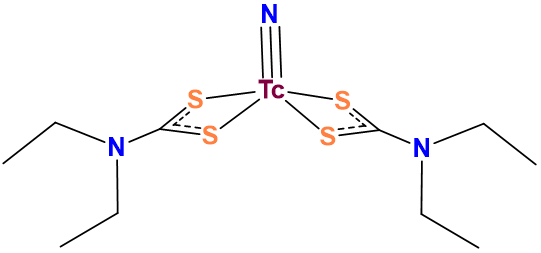
Figure \(\sf{\PageIndex{4}}\). As in this complex, dithiocarbamates commonly bind metals through both sulfur atoms. Consequently, dithiocarbamates are classified as bidentate. this work by Stephen Contakes is licensed under a Creative Commons Attribution 4.0 International License.
Because of these factors it is technically more correct to say that carboxylates usually act as monodentate ligands and dithiocarbamates bidentate ones than it is to say that carboxylates are monodentate ligands and dithiocarbamates bidentate ones. So in other words the ligand classifications presented here are just represent common binding modes.
Exercise \(\PageIndex{1}\)
Determine the denticity of each ligand in the list below and classify them as monodentate, tridentate, etc.
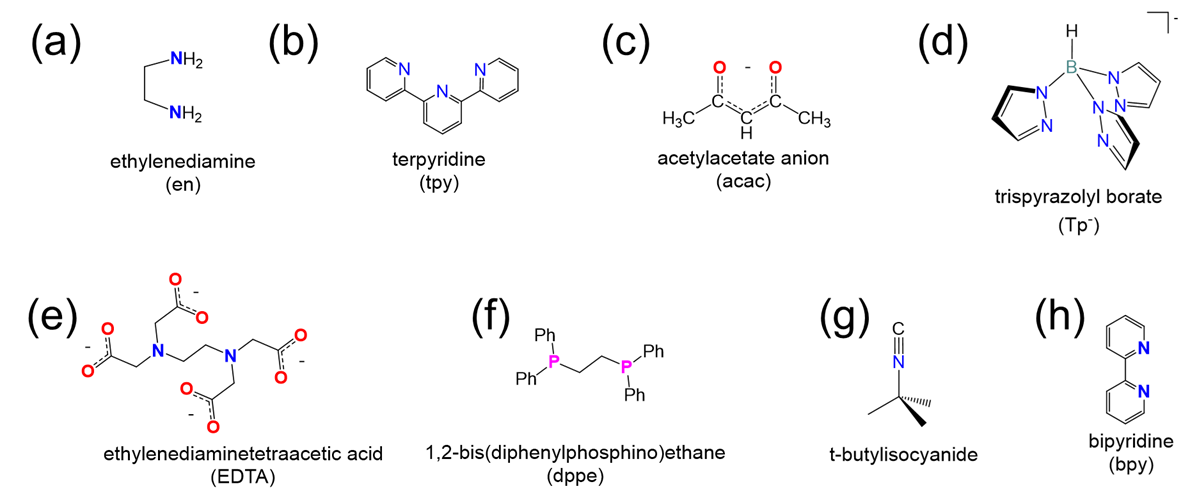
- Answer
-
(a) bidentate
(b) tridentate
(c) bidentate
(d) tridentate (only the lower N on each ring has a lone pair that can be used to bind the metal)
(e) hexadentate (remember that each carboxylate only counts as one point of attachment)
(f) bidentate
(g) monodentate (through the lone pair on the isocyanide C)
(h) bidentate
This experimentally-based classification of dithiocarbamates as bidentate and carboxylates as monodentate can be confusing to a beginner. Fortunately, such experimentally based classifications are embedded in the lists of common monodentate ligands are given in Table \(\sf{\PageIndex{1}}\) and common chelating ligands in Table \(\sf{\PageIndex{2}}\).
A perusal of the ligands in Table \(\sf{\PageIndex{1}}\) reveals that several can bind to a metal in multiple ways. For example, thiocyanate, SCN- can bind metal's through its S or N atoms. Such ligands are called ambidentate ligands. When naming an ambidentate ligand the atom through which it attaches to the metal is commonly specified after the ligand name using the italicized element symbol or, more formally a \(\kappa\) followed by the italicized element symbol.
Table \(\sf{\PageIndex{1}}\). Common monodentate ligands. Most chemists still prefer common names over the IUPAC ones.
| Ligand | Ligand Type | Common name | IUPAC name |
| H | X | hydrido | hydrido |
| F | X | fluoro | fluorido |
| Cl | X | chloro | chlorido |
| Br | X | bromo | bromido |
| I | X | iodo | iodido |
| M-CN | X | cyano | cyanido or cyanido-\(\kappa\)C or cyanido-C |
| RNC | L | alkyl or aryl isocyanide | alkyl or aryl isocyanide |
| N3 | X | azido | azido |
| M-SCN | X | thiocyanato | thiocyanato-\(\kappa\)S or thiocyanato-S |
| M-NCS | X | isothiocyanato | thiocyanato-\(\kappa\)N or thiocyanato-N |
| CH3CO2 | X | acetato | ethanoato |
| N | X3 | nitrido | nitrido |
| NH | X2 | imido | azanediido |
| NH2 | X | amido | azanido |
| NH3 | L | ammine | ammine |
| RNH2, R2NH, R3N | L |
alkylamine, dialkylamine, trialkalyamine (e.g. methylamine for CH3NH2) |
alkylamine, dialkylamine, trialkalyamine (e.g. methylamine for CH3NH2) |
|
piperidine, abbreviated pip |
L |
piperidine |
piperidine |
|
pyridine, abbreviated py |
L |
pyridine |
pyridine |
|
CH3CN acetonitrile, abbreviated MeCN |
L | acetonitrile | acetonitrile |
| P | X3 | phosphido | phosphido |
| PH3 | L | phosphine | phosphane |
| PR3 | L | trialkylphosphine (e.g. trimethylphosphine for Me3P) | trialkylphosphane (e.g. trimethylphosphane for Me3P) |
| PAr3 | L | triarylphosphine (e.g. triphenylphosphine for Ph3P) | triarylphosphine (e.g. triphenylphosphane for Ph3P) |
| O | X2 | oxo | oxido |
| OH | X | hydroxo | hydroxido |
| H2O | L | aqua | aqua |
| S | X2 | sulfo | sulfo |
| HS | X | hydrosulfido | hydrosulfido |
|
RS |
X |
alkanethiolate (e.g. ethanthiolate for EtS-) |
thioalkanoate |
| NO | X | nitrosyl | nitrosyl |
| CO | L | carbonyl | carbonyl |
| CS | L | thiocarbonyl | thiocarbonyl |
| M-NO2 | L | nitro or nitrito-N | nitrito-\(\kappa\)N or nitrito-N |
| M-ONO | X | nitrito or nitrito-O | nitrito-\(\kappa\)O or nitrito-O |
Table \(\sf{\PageIndex{2}}\). Common chelating ligands organized by denticity. Most chemists use the common names and abbreviations to describe these ligands.
|
Common Ligand name |
IUPAC ligand name |
abbreviation (if applicable) |
Ligand Type |
structure or representative/parent structure (shown in the ionization state in which they bind to a metal) |
| bidentate ligands | ||||
| acetylacetonato | 2,4-pentanediono | acac | LX | 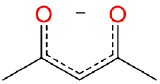 |
|
2,2'-bipyridine |
2,2'-bipyridine |
bpy or bipy |
L2 | 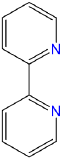 |
| dialkyldithiocarbamato | dialkylcarbamodithiolato |
R2NCS2- or dtc |
LX | 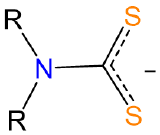 |
|
diphenylphosphinoethane or 1,2-(diphenylphosphino)ethane |
Ethane-1,2-diylbis(diphenylphosphane) |
dppe |
L2 | 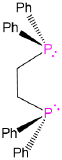 |
|
ethylenediamine |
Ethane-1,2-diamine |
en |
L2 | 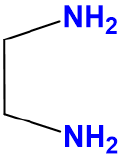 |
|
ethylenedithiolato |
Ethane-1,2-dithiolato |
C2H2S22- |
X2 | 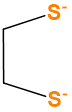 |
| nacnac | N,N'-diphenyl-2,4-pentanediiminato | nacnac | LX | 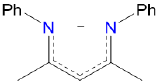 |
| oxalato | oxalato | ox | X2 | 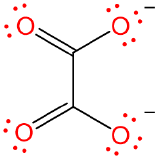 |
|
1,10-phenanthroline or o-phenanthroline |
1,10-phenanthroline |
phen or o-phen |
L2 | 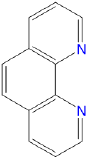 |
| tridentate ligands | ||||
| triazacyclononane | 1,3,7-triazacyclononane | tacn | L3 | 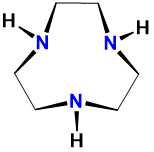 |
| diethylenetriamine | 1,4,7-triazaheptane | dien | L3 | 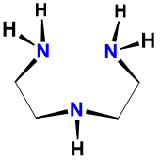 |
|
pyrazoylborato (scorpionate) |
hydrotris(pyrazo-1-yl)borato |
Tp |
L2X | 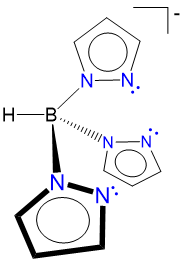 |
|
terpyridine or 2,2';6',2"-terpyridine |
12,22:26,32-terpyridine or 2,6-bis(2-pyridyl)pyridine, tripyridyl, 2,2′:6′,2″-terpyridine |
tpy or terpy |
L3 |  |
| tetradentate ligands | ||||
| \(\beta\), \(\beta\)', \(\beta\)''-triaminotriethylamine | \(\beta\), \(\beta\)', \(\beta\)''-tris(2-aminoethyl)amine | tren | L4 | 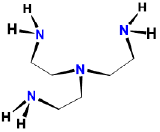 |
| triethylenetetramine | 1,4,7,10-tetraazadecane | trien | L4 | 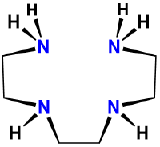 |
| corroles | variable and generally not used | cor or Cor | L2X2 | 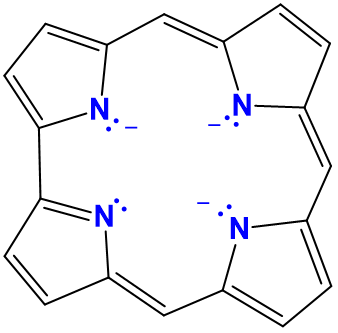 |
| 12-crown-4 | 1,4,7,10-tetraoxacyclododecane | 12-crown-4 | L4 | 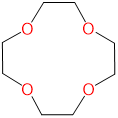 |
|
tetramethylcyclam |
1,4,8,11-tetramethyl-1,4,8,11-tetraazacyclotetradecane |
TMC or cyclam |
L4 | 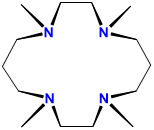 |
| cyclam | 1,4,8,11-tetraazacyclotetradecane | cyclam | L4 |  |
| cyclen | 1,4,7,10-tetraazacyclododecane | cyclen | L4 | 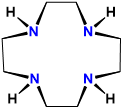 |
| tris(2-pyridylmethyl)amine | 1-pyridin-2-yl-N,N-bis(pyridin-2-ylmethyl)methanamine | tpa or TPA | L4 | 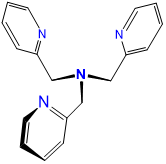 |
| phthalocyanines | variable and generally not used | variable, usually a modified Pc | L2X2 | 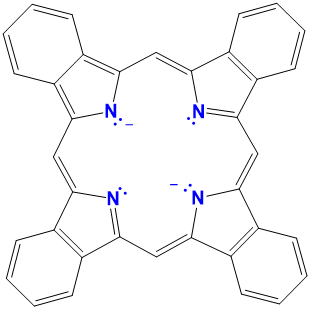 |
|
porphyrins |
variable and generally not used |
variable, usually a modified por, Por, or P (e.g. TPP = tetraphenylporphyrin) |
L2X2 | 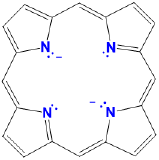 |
| salen | 2,2'-ethylenebis(nitrilomethylidene)diphenoxido | salen | L2X2 |  |
| pentadentate ligands | ||||
|
15-crown-5 |
1,4,7,10,13-Pentaoxacyclopentadecane |
15-crown-5 |
L5 |  |
| tetraethylenepentamine | 1,4,7,10,13-pentaazatridecane | tepa or TEPA | L5 |  |
| hexadentate ligands | ||||
|
18-crown-6 |
1,4,7,10,13,16-hexaoxacyclooctadecane |
18-crown-6 |
L6 |  |
|
2,1,1-cryptand |
4,7,13,18-Tetraoxa- |
2,1,1-crypt or [2.1.1]-cryptand kryptofix 211 and variations thereof |
L6 | 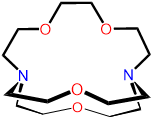 |
|
ethylenediaminetetraaceto |
2,2′,2″,2‴-(Ethane-1,2-diyldinitrilo)tetraaceto |
EDTA, edta, Y4- |
L2X4 | 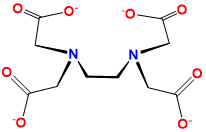 |
| heptadentate ligands | ||||
|
2,2,1-cryptand |
4,7,13,16,21-pentaoxa- |
2,2,1-crypt or [2.2.1]-cryptand kryptofix 221 and variations thereof |
L7 | 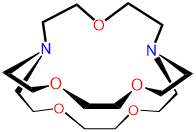 |
| octadentate ligands | ||||
|
2,2,2-cryptand |
4,7,13,16,21,24-Hex |
2,2,2-crypt or [2.2.2]-cryptand kryptofix 222 and variations thereof |
L8 | 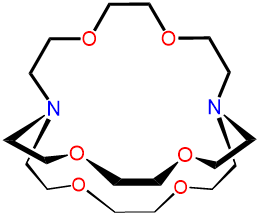 |
|
pentetato acid or diethylenetriaminepentaacetato or DTPA |
2-[bis[2-[bis(carboxylatomethyl)amino]ethyl]amino]acetato |
DTPA |
L3X5 | 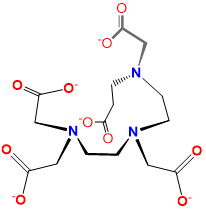 |
|
DOTA or tetraxetan |
1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid |
Dota, DOTA |
L4X4 | 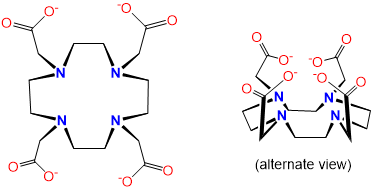 |
Rules for Naming Coordination Compounds
There are well-established rules for both naming and writing the formulae of coordination compounds. The purpose of these rules is to facilitate clear and precise communication among chemists. As with all such rules some are more burdensome than others to employ and some serve more crucial roles in the communication process while others are more peripheral.
Coordination Complexes are named as the ligand derivatives of a metal
A variety of systems have been used for naming coordination compounds since the development of the discipline in the time of Alfred Werner. In this section the most common approaches as they are currently used by practicing chemists will be described. Those who need a more thorough and accurate acquaintance with the full IUPAC nomenclature rules are encouraged to consult the IUPAC brief guide to inorganic nomenclature followed by complete guidelines, commonly known as the IUPAC red book. The systems for naming coordination compounds used at present are additive, meaning that they consider coordination compounds as comprised of a central metal to which are added ligands. To specify the structure and bonding in this metal-ligand complex then involves
1. When there are several different ways of attaching the metal and ligands, specifying the structural or stereoisomer
2. systematically listing the ligands in a way that, as necessary, conveys information about how they are linked to the metal and their stereochemistry
3. providing the identity of the metal and its valence, or if the valence is unclear, at least the overall charge on the complex
4. specifying any counterions present
Since the stereochemistry of coordination compounds forms is the subject subsequent sections, in this section it will be addressed by simply giving the prefixes that designate stereochemistry as if they were self-evident. Do not worry about these for now. They will make sense after you have learned more about stereochemistry in the next sections. At that time, you can go back over the examples in this section to solidify your understanding of how to name coordination compounds.
Before going into these rules it is worth pointing out a few things.
1. It is easiest to learn these rules by starting with one or two of the rules, learning how to apply them, and then adding additional rules one at a time.
2. The rules also assume some familiarity with common coordination geometries and patterns of isomerism in metal complexes. Again, we will learn more about these in a later section. For now it is safe to assume
- complexes in which the metal has a coordination number of six are octahedral
- complexes in which the metal has a coordination number of five are trigonal bipyramidal
- complexes in which PtII , PdII , or RhI, or IrI have a coordination number of four are square planar
- other complexes in which the metal has a coordination number of four are tetrahedral
Like all assumptions these don't always work in real life but they should be good enough for now.
Rule 1: If ions are present, name the cation first, followed by the anion.
Examples:
K2[PtIICl4]: potassium tetrachloroplatinate(2- or II)
[CoIII(NH3)6](NO3)3: hexaamminecobalt(3+ or III) nitrate
[CoIII(NH3)6][CrIII(C2O4)3]: hexaamminecobalt(3+ or III) tris(oxalato)chromate(3- or III)
You may notice two unusual features in these name. First, there are two numbers in parentheses. Second, in a few places the name of the metal seems to have changed to end in -ate. Both of these will be addressed in later rules.
Rule 2: When multiple isomers are possible, designate the particular isomer in italics at the front of the name of each complex
When a complex might exist as one of two stereosisomers prefixes are commonly used to designate which isomer is present. The most common cases are listed in Table \(\sf{\PageIndex{3}}\).
Table\(\sf{\PageIndex{3}}\). Prefixes used to specify isomerism about a metal center when naming and writing coordination compounds' formulae.
| Type of isomerism | Graphical reminder | Prefixes |
|
Geometric, cis- , trans- |
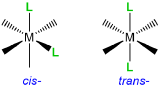 |
cis- or trans- |
|
Geometric, fac- /mer- |
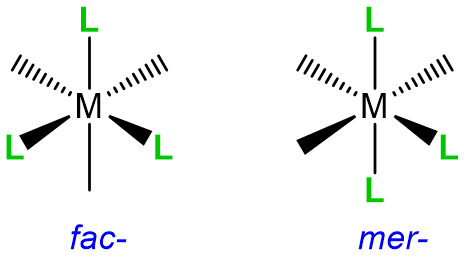 |
fac- or mer- |
|
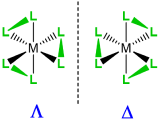
Enantiomers, \(\Lambda\)-, \(\Delta\)- |
|
\(\Lambda\)- or \(\Delta\)- |
Examples of how isomerism about a metal center is designated are given in Figure \(\sf{\PageIndex{5}}\).
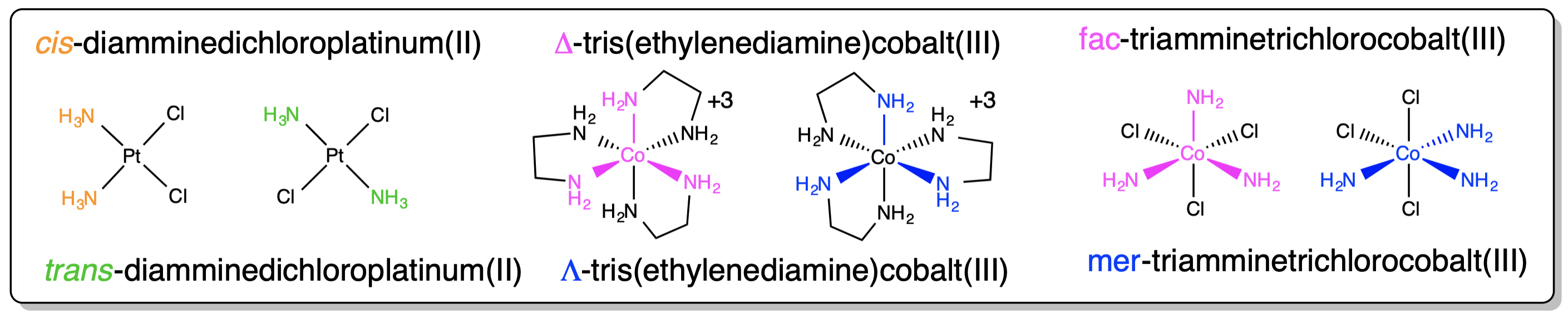
Figure \(\sf{\PageIndex{5}}\). Application of nomenclature rules for stereosimerism about a metal.1
There are a number of other cases where it might be advisable to specify the stereochemistry of a complex. These cases involve specifying
- the coordination geometry about a metal center (octahedral, trigonal prismatic, tetrahedral, square planar, etc. )
- the geometry cannot be unambigouously described by a single cis/trans or fac/mer relaionship of ligands
These cases may also be handled by using a designator to specify the coordination geometry and, as necessary, giving the position of ligated atoms in terms of designated numbered positions for that geometry. See the IUPAC red book for details as such cases fall outside the scope of this course.
Rule 3: Specify the identity, number, and as appropriate, isomerism of the ligands present in alphabetical order by ligand name.
Before specifying the metal, the ligands are written as prefixes of the metal.
In specifying the ligands several rules are followed.
- The ligands are written in alphabetical order by the ligand name only; symbols are not considered and prefixes do not count in determining alphabetical order.
Example: In the name of the complex ion [Co(NH)3Cl]2+, pentamminechlorocobalt(II), the ammine ligand is named before the chloro ligand because the order is alphabetical by the ligand name by virtue of which ammine comes before chloro.
- Prefixes are used to indicate the number of each ligand present. Specifically, di-, tri-, tetra- , penta-, hexa-, etc. prefixes are used to indicate multiple ligands of the same type EXCEPT when the ligand is polydentate or its name already has a di-, tri-, tetra- etc. In that case bis-,tris-, tetrakis-, etc. are used instead. These prefix rules are summarized in Table \(\sf{\PageIndex{3}}\).
Table \(\sf{\PageIndex{3}}\). Prefixes used to specify the number of a given ligand present.
| Number of identical ligands | prefix used when the ligand name is simple | prefix used when the ligand is polydentate or its name already has a di-, tri-, tetra- etc. |
| 2 | di- | bis- |
| 3 | tri- | tris- |
| 4 | tetra- | tetrakis- |
| 5 | penta- | pentakis- |
| 6 | hexa- | hexakis- |
| 7 | hepta- | heptakis- |
| 8 | octa- | octakis- |
| 9 | nona- | nonakis- |
| 10 | deca- | decakis- |
An example of the application of the prefix rule is given in Figure \(\sf{\PageIndex{6}}\).
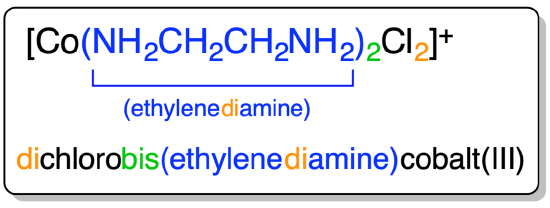
Figure \(\sf{\PageIndex{6}}\). Example of the use of prefixes to specify the number of ligands of each type in a complex.1
- Ligand names are based on their charge
- L-type ligand names are the same as the names of their neutral compounds with two caveats
- names that involve spaces should either be put in parentheses or the spaces should be eliminated (preferred)
Example: cis-dichlorobis(dimethyl sulfoxide)platinum(II) or cis-dichlorobis(dimethylsulfoxide)platinum(II)
- A few ligands are given common names.
- H2O = aqua
- NH3 = ammine (notice that there ate two n's)
- CO = carbonyl
- CS = thiocarbonyl
- NO = nitrosyl
- For X-type ligands, the ending is typically changed to end in an -”o”
Examples: Cl = chloro, NH2 = amido, N3 = azido
Caveat: some X-type ligands have common names that may also be used
Examples:
I = iodo or iodino
CN = cyano or cyanido
O = oxo or oxido
The IUPAC and common names of many ligands are given in Tables \(\sf{\PageIndex{1}}\). and \(\sf{\PageIndex{2}}\).
- When an ambidentate ligand is present the atom through which it is bound to the metal is indicated by giving either its element symbol or a \(\kappa\) and its element symbol in italics after the ligand name
Example:
M-SCN is thiocyanato-S or thiocyanato-\(\kappa\)S
M-NCS = thiocyanato-N or thiocyanato-\(\kappa\)N
The use of \(\kappa\) and an element symbol to indicate how a ligand and metal are linked is called a k-term. More complex k-terms might also involve specifying the atoms by number, though their use is outside the scope of this text.
- As appropriate, additional information about the way a ligand is bound to the metal center and/or its stereochemistry is specified using a prefix. The prefixes to provide linkage and stereochemistry for ligands are given in Table\(\sf{\PageIndex{4}}\).
Table\(\sf{\PageIndex{4}}\). Prefixes used to specify ligands' isomerism when naming and writing coordination compounds' formulae. Some of these types of isomerism will be discussed in later pages.
| Type of isomerism | Graphical reminder | Prefixes |
|
when a multidentate ligand binds through less than the full number of atoms |
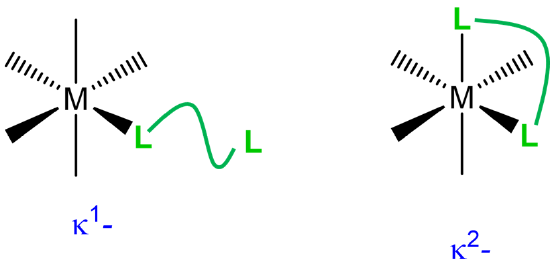 |
\(\kappa\)n where n is the number of attached atoms; used when the attached atoms are not directly connected by a chemical bond. The metal-ligand bonding usually involves \(\sigma\)-type coordination. |
|
bridging ligands |
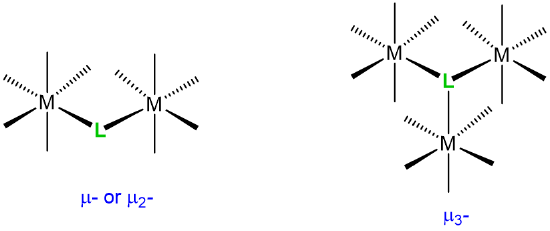 |
\(\mu\)n where n is the number of atoms bridged. The number n is usually omitted when n =2. |
|
chelating ligand ring twist |
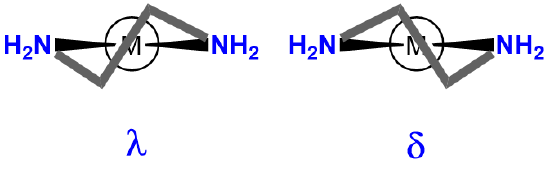 |
\(\lambda\)- or \(\delta\)- |
A example showing how the nomenclature rule is applied to a ligand that can have two coordination modes is given in Figure \(\sf{\PageIndex{7}}\).

Figure \(\sf{\PageIndex{7}}\). Use of the \(\kappa\) notation to specify the number of attached groups in a multidentate ligand.
- If desired, parentheses may be used to delineate a ligand name to make it easier to identify in the name. This can be particularly helpful when the name contains a lot of information to keep track of. An example is given in Figure \(\sf{\PageIndex{8}}\).
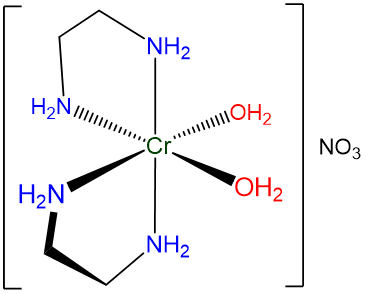
Figure \(\sf{\PageIndex{8}}\). When naming the complex shown cis-diaquabis(ethylenediamine)chromium(III) nitrate is easier to read than cis-diaquabisethylenediaminechromium(III) nitrate.
Rule 4: Specify the identity of the metal
- In neutral and cationic complexes the metal's name is used directly
- e.g. as in hexammineruthenium(III) for [Ru(NH3)6]3+
- In anionic complexes, -ate replaces -ium, -en, or –ese or adds to the metal name.
e.g. as in hexachloromanganate(IV) for [MnCl6]2-
- In anionic complexes of some metals a Latin-derived name is used instead of the element's English name. These names are given in Table \(\sf{\PageIndex{5}}\).
Table \(\sf{\PageIndex{5}}\). Latin terms for Select Metal Ions. Redrawn from this page describing the nomenclature of coordination complexes.
| Transition Metal | Latin |
|---|---|
| Copper | Cuprate |
| Gold | Aurate |
| Iron | Ferrate |
| Lead | Plumbate |
| Silver | Argentate |
| Tin | Stannate |
An example of the application of the metal naming rules is given in Figure \sf{\PageIndex{9}}\).

Figure \(\sf{\PageIndex{9}}\). Example of the application of the metal specification rules to a cationic and anionic platinum complexes.1
Rule 5: Specify the valence of the metal.
Two different systems are used to specify the valence of the metal.
- In the Stock system the metal's valence is indicated in Roman numerals after the metal name.
Examples:
[CoCl(NH3)5]Cl2 = pentamminechlorocobalt(III) chloride
[PtBr2(bpy)] = bipyridinedibromoplatinium(II)
K[Ag(SCN)2] = potassium di-S-thiocyanatoargentate(I)
- In the Ewing-Bassett system the charge on the complex is specified in Arabic numerals after the complex name. This provides a way of specifying a complex even when the valence of the metal isn't known and, in cases where it is known, the value of the metal's valence may be inferred from the complex ion's charge.
[CoCl(NH3)5]Cl2 = pentamminechlorocobalt(2+) chloride
[PtBr2(bpy)] = bipyridinedibromoplatinium(0)
K[Ag(SCN)2] = potassium di-S-thiocyanatoargentate(1-)
Exercise \(\PageIndex{2}\). Assigning metal valence in a complex
In order to name a complex in the Stock system it is necessary to assign a valence to the metal.
For this reason it is important to be able to assign the valence of a metal in a complex. Remember the relationship
valence - number of X-type ligands = total charge on the complex
Assign the valence of the metal in the following real and hypothetical complexes.
- K3[Fe(CN)6]
- K2[PtCl4]
- [MnCl(por)]
- [Ru(bpy)3]Cl2
- [PdCl2(dppe)]
- Answer for K3[Fe(CN)6].
-
This contains [Fe(CN)6]3-; so valence - 6 (for 6 CN-) = -3 (the complexes' charge) so valence = +3 or Fe3+.
- Answer for K2[PtCl4].
-
This contains [PtCl4]2-; so valence - 4 (for 4 Cl) = -2 (the complexes' charge) so valence = +2 or Pt2+.
- Answer for [MnCl(por)].
-
valence - 2 (for por a which is L2X2; see table 9.2.2) - 1 (for Cl) = 0 (the complexes' charge) so valence = +3 or Mn3+.
- Answer [Ru(bpy)3]Cl2.
-
This contains [Ru(bpy)3]2+; so valence - 0 (for bpy which is L2) = +2 (the complexes' charge) so valence = +2 or Ru2+.
- Answer [PdCl2(dppe)].
-
valence - 2 (for 2 Cl) - 0 (for dppe which is L2) = 0 (the complexes' charge) so valence = +2 or Pd2+.
Exercise \(\PageIndex{3}\): Simple Nomenclature Problems.
Name the following compounds in both the Stock and Ewing-Bassett systems:
- [Ru(NH3)6](NO3)3
- K2[PtCl4]
- K[Ag(CN)2]
- Cs[CuBrCl2F]
- [Cu(acac)2]
- K4[Fe(CN)6]
- trans-[Cu(en)2(NO2)2] (the N is bound to Cu)
- cis-IrCl2(CO)(PPh3) (ignore stereochemistry)
- IrCl(PPh3)
- Answer
-
Complex Stock system name Ewing-Bassett System name a [Ru(NH3)6](NO3)3 hexammineruthenium(III) nitrate hexammineruthenium(3+) nitrate b K2[PtCl4] potassium tetrachloroplatinate(II) potassium tetrachloroplatinate(2-) c K[Ag(CN)2] potassium dicyanoargentate(I) potassium dicyanoargentate(1-) d Cs2[CuBrCl2F] cesium bromodichloroflourocuprate(II) cesium bromodichloroflourocuprate(2-) e [Cu(acac)2] bis(acetylacetonato)copper(II) bis(acetylacetonato)copper(0) f K4[Fe(CN)6] potassium hexacyanoferrate(II) or potassium hexacyanidoferrrate(II) potassium hexacyanoferrate(4-) or potassium hexacyanidoferrrate(4-) g trans-[Cu(en)2(NO2)2] (the N is bound to Cu) bis(ethylenediamine)bisnitrocopper(II) or bis(ethylenediamine)bis(nitrito-\(\kappa\)N)copper(II) bis(ethylenediamine)bisnitrocopper(0) or bis(ethylenediamine)bis(nitrito-\(\kappa\)N)copper(0) h cis-IrCl2(CO)(PPh3)
cis-dichlorocarbonyltriphenylphosphineiridium(I)
or cis-dichloro(carbonyl)(triphenylphosphine)iridium(I)
cis-dichlorocarbonyltriphenylphosphineiridium(0)
or cis-dichloro(carbonyl)(triphenylphosphine)iridium(0)
i IrCl(PPh3) chlorotris(triphenylphosphine)iridium(I) chlorotris(triphenylphosphine)iridium(0)
Exercise \(\PageIndex{8}\)
Draw structural formulae for the following compounds and ions. You may assume that
- complexes in which the metal has a coordination number of six are octahedral
- complexes in which the metal has a coordination number of five are trigonal bipyramidal
- complexes in which PtII , PdII , or RhI, or IrI have a coordination number of four are square planar
- other complexes in which the metal has a coordination number of four will be tetrahedral
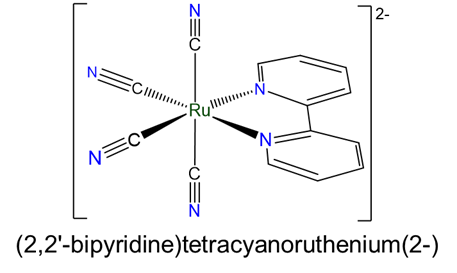
- (2,2'-bipyridine)tetracyanoruthenium(2-)
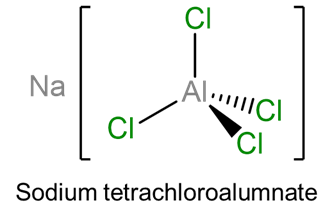
- sodium tetrachloroalumnate (note that since Al is a main group metal with a generally fixed oxidation state no oxidation state is given)
- carbonylhydridotris(triphenylphosphine)rhodium(I) (the ligands in this complex occupy sterically preferred positions)
- bromotrichlorocobaltate(III)
- sodium tris(oxalato)cobalt(III)
- fac-(1,10-phenanthroline)tricarbonylchlororhenium(I)
- mer-triaquatrichlorochromium(III)
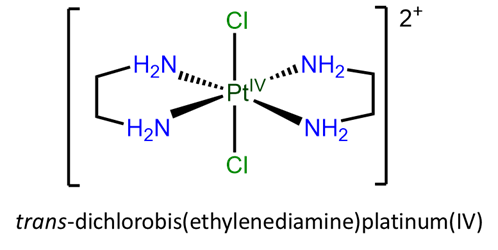
- trans-dichlorobis(ethylenediamine)platinum(IV)
- Answers
- a
-
- b
-
- c
-
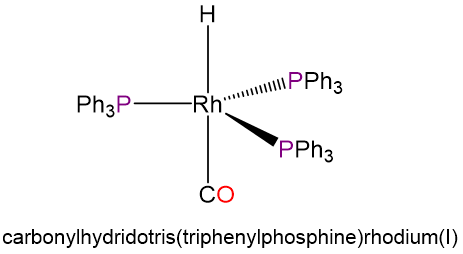
- d
-
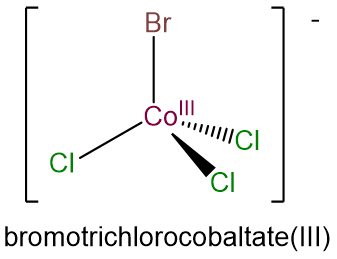
- e
-
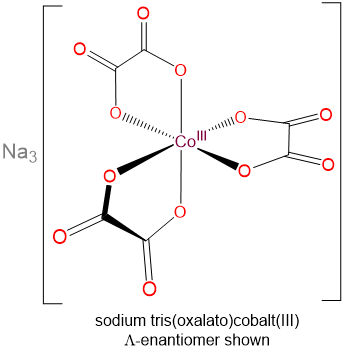
- f
-
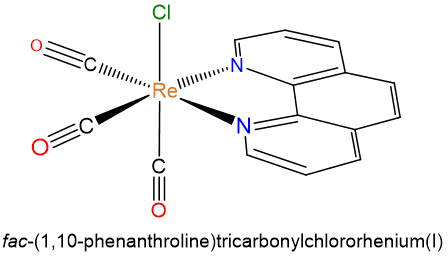
- g
-
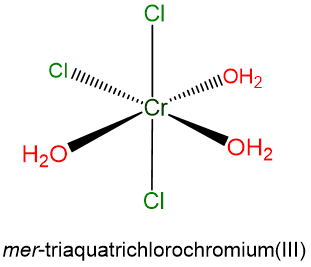
- h
-
Note \(\PageIndex{1}\): Sometimes the most helpful name to give a compound is 42.
Even though the IUPAC nomenclature rules permit specification of even the most complex structures, it is often much easier and more effective to supply a numbered structure that can be referred to instead of the IUPAC name. Consider bis{[(μμ-2-mercaptoethyl)(2-mercaptoethyl)-methylthioethylaminato (2-)]Nickel(II)}. Which is easier, to expect readers and hearers to work out the structure from that name or to just refer them to compound 42 in Figure \(\PageIndex{10}\).
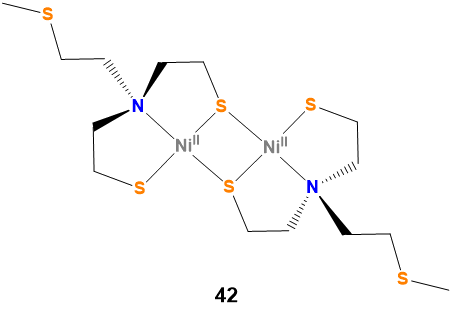
Figure \(\PageIndex{10}\). Structure of bis{[(μ-2-mercaptoethyl)(2-mercaptoethyl)-methylthioethylaminato (2-)]Nickel(II)}. The authors of the synthesis of this compound in Inorganic syntheses2 may have had to figure out an IUPAC name for this compound but if you have this scheme in your paper and your instructor is OK with it you can just call it 42.
References
1. Haas, K. Naming Transition Metal Complexes. https://chem.libretexts.org/Courses/Saint_Mary's_College%2C_Notre_Dame%2C_IN/CHEM_342%3A_Bio-inorganic_Chemistry/Readings/Week_2%3A_Introduction_to_Metal-Ligand_Interactions_and_Biomolecules/2.1_Transition_metal_complexes/2.1.6%3A_Naming_Transition_Metal_Complexes
2. The structure and name is taken from Choudhury, S. B.; Allan, C. B.; Maroney, M.; Wodward, A. D.; Lucas, C. R. Inorg. Synth. 1998, 32, 98-107.
Contributors and Attributions
Stephen Contakes, Westmont College, to whom comments, corrections, and criticisms should be addressed.
with some examples taken from Naming Transition Metal Complexes by Kathryn Haas.
Consistent with the policy for original artwork made as part of this project, all unlabeled drawings of chemical structures are by Stephen Contakes and licensed under a Creative Commons Attribution 4.0 International License.