5.1: Introduction to Coordination Chemistry
- Page ID
- 296074
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Coordination chemistry is the study of the compounds that form between metals and ligands, where a ligand is any molecule or ion that binds to the metal. A metal complex is the unit containing the metal bound to its ligands. For example, [PtCl2(NH3)2] is the neutral metal complex where the Pt(II) metal is bound to two Cl ligands and two NH3 ligands. If a complex is charged, it is called a complex ion (ex. [Pt(NH3)4]+2 is a complex cation). A complex ion is stabilized by formation of a coordination compound with ions of opposite charge (ex. [Pt(NH3)4]Cl2). It is convention to write the formula of a complex or complex ion inside of square brackets, while counterions are written outside of the brackets. In this convention, it is understood that ligands inside the brackets are bound directly to the metal, in the metal's first coordination sphere (a.k.a inner coordination sphere). Ions written outside of the brackets are assumed to be in the second coordination sphere, and they are not directly bound to the metal.
- Neutral Complex: [CoCl3(NH3)3]
- Complex Cation: [Co(NH3)6]3+
- Complex Anion: [CoCl4(NH3)2]-
- Coordination Compound: K4[Fe(CN)6]
A common metal complex is [Ag(NH3)2]+ formed when Ag+ ions are mixed with neutral ammonia molecules.
\[Ag^+ + 2 NH_3 \rightarrow [Ag(NH_3)_2]^+\]
A complex [Ag(S2O3)2]3- is formed between silver ions and negative thiosulfate ions:
\[Ag^+ + 2 S_2O_3^{2-} \rightarrow [Ag(S_2O_3)_2]^{3-}\]
How did the study of coordination compounds started?
The coordination chemistry was pioneered by Nobel Prize winner Alfred Werner (1866-1919). He received the Nobel Prize in 1913 for his coordination theory of transition metal-amine complexes. At the start of the 20th century, inorganic chemistry was not a prominant field until Werner studied the metal-amine complexes of cobalt. Werner recognized the existence of several forms of cobalt-ammonia chloride. These compounds have different color and other characteristics. The chemical formula has three chloride ions per mole, but the number of chloride ions that precipitate with Ag+ ions per formula was not always three. He thought only ionized chloride ions will form precipitate with silver ion. In the following table, the number below the Ionized Cl- is the number of ionized chloride ions per formula. To distinguish ionized chloride from the coordinated chloride, Werner formulated the Complex formula and explained structure of the cobalt complexes.
| Solid | Color | Ionized Cl- | Complex formula |
|---|---|---|---|
| CoCl36NH3 | Yellow | 3 | [Co(NH3)6]Cl3 |
| CoCl35NH3 | Purple | 2 | [Co(NH3)5Cl]Cl2 |
| CoCl34NH3 | Green | 1 | trans-[Co(NH3)4Cl2]Cl |
| CoCl34NH3 | Violet | 1 | cis-[Co(NH3)4Cl2]Cl |
The structures of the complexes were proposed based on a coordination number of 6. The six ligands can be ammonia or chloride. Two different structures were proposed for the last two compounds, the trans- compound has two chloride ions on opposite vertices of an octahedral, whereas the the two chloride ions are adjacent to each other in the cis- compound. The cis- and trans- compounds are known as geometric isomers. Isomers will be the focus of a later section.
Classification of ligands
The discussion of the cobalt complexes above suggests that there are two types of chlorides in the [Co(NH3)5Cl]Cl2 and cis-/trans-[Co(NH3)4Cl2]Cl complexes. In comparing the formula and the amounts of ionized Cl- listed in Table \(\sf{\PageIndex{1}}\), the outer sphere chlorides must be the ionizable ones. The reason the inner sphere ligands are not ionizable is because there is significant covalent character in the bond with the cobalt. Even though we call these inner sphere ligands chloride (or more properly chloro) ligands, they are not Cl-, but rather better thought of as being Co-Cl.
In order to account for this difference, we will use the Covalent Bond Classification (CBC) method for thinking about ligands. In this system, ligands are classified as either being X-type (one-electron donors) or L-type (two-electron donors). In order to determine if a ligand is an X-type or L-type ligand, perform a thought experiment in which you remove the ligand from the metal as a neutral atom or molecule.
Example \(\PageIndex{1}\)
Consider the complex cation, [Co(NH3)5Cl]2+. What types of ligands are NH3 and Cl?
NH3
There are three possible ways you could think of breaking the Co-N bond. One could consider homolytic cleavage (one electron goes with Co and one with N) or heterolytic cleavage (one atom takes both electrons). The homolytic cleavage option is shown in the middle results in the formation of NH3+. Our goal is to remove the ligand as a neutral species, so this is not the correct option. If both electrons in the bond go with the nitrogen, there is no charge on the outgoing ligand. When considering the two heterolytic cleavage options, the pair of electrons can either go with the nitrogen (left) or Co (right). Only one of these options (the one on the left) results in the formation of the neutral NH3 molecule. As such, NH3 is a two-electron donor and therefore a L-type ligand.
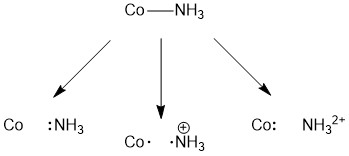
It is important to note two things before we move on to our consideration of chloride. First, even though there are four bonds to the nitrogen atom in the initial Co-NH3, we do not put a positive charge on the nitrogen. While the nitrogen atom bound to cobalt is certainly less electron rich than the nitrogen atom in an uncoordinated ammonia molecule, if we think back on our discussion of the acidity of hexaaqua species, the charge is difficult to localize and so we ignore it. This will be true for all metal complexes. Second, no charge is shown on any of the cobalt species as accounting for charge on the ligand will likely be more straightforward.
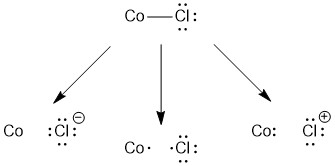
Cl
Similar to our treatment of NH3, there are three ways to consider removing the chloride. In this case, both heterolytic cleavage options (left and right) result in a charged chlorine species so these are not the correct option. However homolytic cleavage (center) of the Co-Cl bond results in a neutral chlorine atom. So the chloride ligand is a X-type ligand.
Why do we need to classify the different ligand types? Doing so is going to help us in determining the valence of the metal. Valence is somewhat similar to oxidation state, but there are subtle differences. The main difference is that valence accounts for both the overall charge on the complex and the number of X-type ligands in the complex. The emphasis with valence is once again on the idea that the metal needs to provide electrons to make covalent bonds to some ligands. To determine the valence of a metal use the following equation:
valence - number of X-type ligands = overall charge on the complex
Example \(\PageIndex{2}\)
Determine the valence of cobalt in [Co(NH3)5Cl]2+.
Previously we determined that the cobalt in this complex has one X-type ligand, the chloride. The complex has an overall +2 charge. Based on the equation above, the valence of the cobalt would be +3 or Co(III).
Had you followed traditional oxidation state rules, you should have arrived at the same conclusion. These rules would have treated the chloride as Cl- and accounted for the remainder of the charge by oxidizing the cobalt. While a subtle difference, the results of experiments in which Ag+ is added to [Co(NH3)5Cl]2+ clearly indicate that the chloride is not ionizable and therefore should not be treated as Cl-. While similar, the idea of valence emphasizes the covalent nature of the Co-Cl bond.
Exercise \(\PageIndex{1}\)
Determine the valence of cobalt in [Co(NH3)4Cl2]Cl.
- Answer
-
While the amounts have changed, the NH3 ligands are still L-type and the Cl ligands are X-type.
The valence on cobalt is (valence - 2 = +1) or Co(III) just like in the previous example.
Contributors and Attributions
Chung (Peter) Chieh (Professor Emeritus, Chemistry @ University of Waterloo)

