5.6: Relating Structure to Acidity and Basicity
- Page ID
- 364674
- Rationalize trends in acid–base strength in relation to molecular structure
Strong acids, such as \(\ce{HCl}\), \(\ce{HBr}\), and \(\ce{HI}\), all exhibit the same strength in water. The water molecule is such a strong base compared to the conjugate bases Cl−, Br−, and I− that ionization of these strong acids is essentially complete in aqueous solutions. In solvents less basic than water, we find \(\ce{HCl}\), \(\ce{HBr}\), and \(\ce{HI}\) differ markedly in their tendency to give up a proton to the solvent. For example, when dissolved in ethanol (a weaker base than water), the extent of ionization increases in the order \(\ce{HCl < HBr < HI}\), and so \(\ce{HI}\) is demonstrated to be the strongest of these acids. The inability to discern differences in strength among strong acids dissolved in water is known as the leveling effect of water.
Water also exerts a leveling effect on the strengths of strong bases. For example, the oxide ion, O2−, and the amide ion, \(\ce{NH2-}\), are such strong bases that they react completely with water:
\[\ce{O^2-}(aq)+\ce{H2O}(l)⟶\ce{OH-}(aq)+\ce{OH-}(aq)\]
\[\ce{NH2-}(aq)+\ce{H2O}(l)⟶\ce{NH3}(aq)+\ce{OH-}(aq)\]
Thus, O2− and \(\ce{NH2-}\) appear to have the same base strength in water; they both give a 100% yield of hydroxide ion.
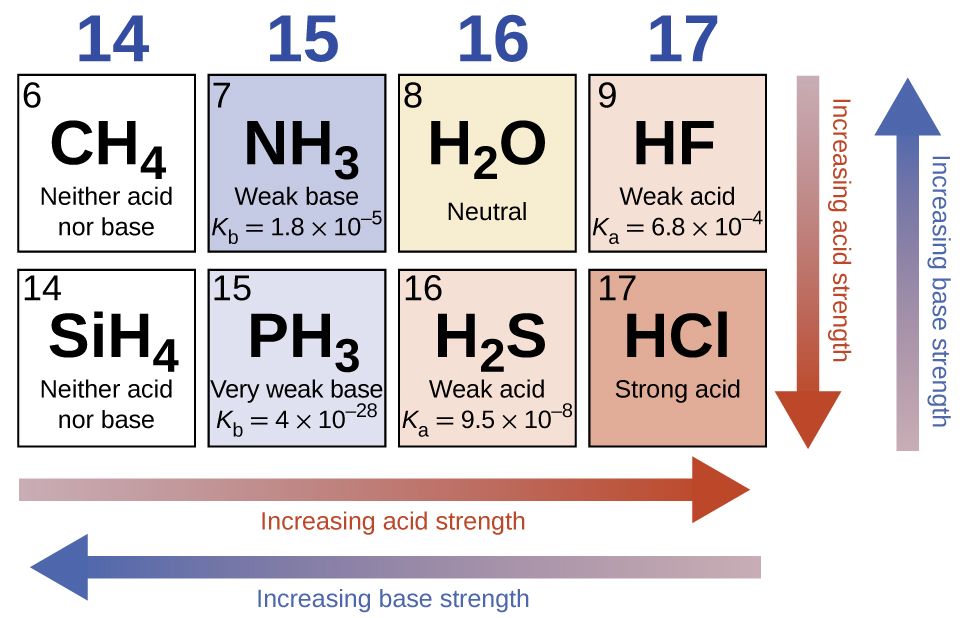
In the absence of any leveling effect, the acid strength of binary compounds of hydrogen with nonmetals (A) increases as the H-A bond strength decreases down a group in the periodic table. For group 17, the order of increasing acidity is \(\ce{HF < HCl < HBr < HI}\). Likewise, for group 16, the order of increasing acid strength is H2O < H2S < H2Se < H2Te. Across a row in the periodic table, the acid strength of binary hydrogen compounds increases with increasing electronegativity of the nonmetal atom because the polarity of the H-A bond increases. Thus, the order of increasing acidity (for removal of one proton) across the second row is \(\ce{CH4 < NH3 < H2O < HF}\); across the third row, it is \(\ce{SiH4 < PH3 < H2S < HCl}\) (see Figure \(\PageIndex{1}\)).
Compounds containing oxygen and one or more hydroxyl (OH) groups can be acidic, basic, or amphoteric, depending on the position in the periodic table of the central atom E, the atom bonded to the hydroxyl group. Such compounds have the general formula OnE(OH)m, and include sulfuric acid, \(\ce{O2S(OH)2}\), sulfurous acid, \(\ce{OS(OH)2}\), nitric acid, \(\ce{O2NOH}\), perchloric acid, \(\ce{O3ClOH}\), aluminum hydroxide, \(\ce{Al(OH)3}\), calcium hydroxide, \(\ce{Ca(OH)2}\), and potassium hydroxide, \(\ce{KOH}\):
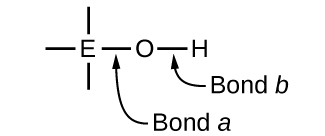
If the central atom, E, has a low electronegativity, its attraction for electrons is low. Little tendency exists for the central atom to form a strong covalent bond with the oxygen atom, and bond a between the element and oxygen is more readily broken than bond b between oxygen and hydrogen. Hence bond a is ionic, hydroxide ions are released to the solution, and the material behaves as a base—this is the case with Ca(OH)2 and KOH. Lower electronegativity is characteristic of the more metallic elements; hence, the metallic elements form ionic hydroxides that are by definition basic compounds.
If, on the other hand, the atom E has a relatively high electronegativity, it strongly attracts the electrons it shares with the oxygen atom, making bond a relatively strongly covalent. The oxygen-hydrogen bond, bond b, is thereby weakened because electrons are displaced toward E. Bond b is polar and readily releases hydrogen ions to the solution, so the material behaves as an acid. High electronegativities are characteristic of the more nonmetallic elements. Thus, nonmetallic elements form covalent compounds containing acidic −OH groups that are called oxyacids.
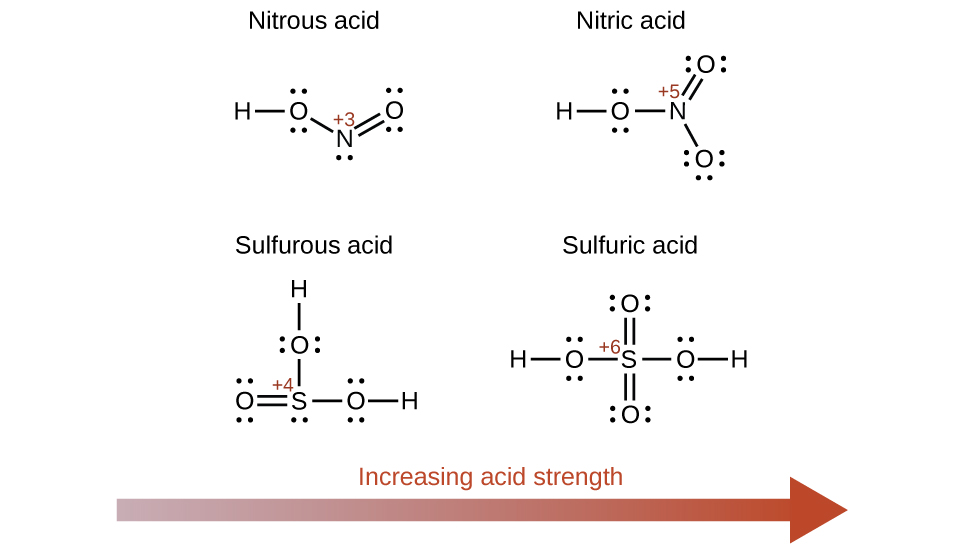
Increasing the oxidation number of the central atom E also increases the acidity of an oxyacid because this increases the attraction of E for the electrons it shares with oxygen and thereby weakens the O-H bond. Sulfuric acid, H2SO4, or O2S(OH)2 (with a sulfur oxidation number of +6), is more acidic than sulfurous acid, H2SO3, or OS(OH)2 (with a sulfur oxidation number of +4). Likewise nitric acid, HNO3, or O2NOH (N oxidation number = +5), is more acidic than nitrous acid, HNO2, or ONOH (N oxidation number = +3). In each of these pairs, the oxidation number of the central atom is larger for the stronger acid (Figure \(\PageIndex{2}\)).
Hydroxy compounds of elements with intermediate electronegativities and relatively high oxidation numbers (for example, elements near the diagonal line separating the metals from the nonmetals in the periodic table) are usually amphoteric. This means that the hydroxy compounds act as acids when they react with strong bases and as bases when they react with strong acids. The amphoterism of aluminum hydroxide, which commonly exists as the hydrate \(\ce{Al(H2O)3(OH)3}\), is reflected in its solubility in both strong acids and strong bases. In strong bases, the relatively insoluble hydrated aluminum hydroxide, \(\ce{Al(H2O)3(OH)3}\), is converted into the soluble ion, \(\ce{[Al(H2O)2(OH)4]-}\), by reaction with hydroxide ion:
\[[\ce{Al(H2O)3(OH)3}](aq)+\ce{OH-}(aq)⇌\ce{H2O}(l)+\ce{[Al(H2O)2(OH)4]-}(aq) \nonumber\]
In this reaction, a proton is transferred from one of the aluminum-bound H2O molecules to a hydroxide ion in solution. The \(\ce{Al(H2O)3(OH)3}\) compound thus acts as an acid under these conditions. On the other hand, when dissolved in strong acids, it is converted to the soluble ion \(\ce{[Al(H2O)6]^3+}\) by reaction with hydronium ion:
\[\ce{3H3O+}(aq)+\ce{Al(H2O)3(OH)3}(aq)⇌\ce{Al(H2O)6^3+}(aq)+\ce{3H2O}(l) \nonumber\]
In this case, protons are transferred from hydronium ions in solution to \(\ce{Al(H2O)3(OH)3}\), and the compound functions as a base.
Summary
The strengths of the binary acids increase from left to right across a period of the periodic table (CH4 < NH3 < H2O < HF), and they increase down a group (HF < HCl < HBr < HI). The strengths of oxyacids that contain the same central element increase as the oxidation number of the element increases (H2SO3 < H2SO4). The strengths of oxyacids also increase as the electronegativity of the central element increases [H2SeO4 < H2SO4].
Glossary
- leveling effect of water
- any acid stronger than \(\ce{H3O+}\), or any base stronger than OH− will react with water to form \(\ce{H3O+}\), or OH−, respectively; water acts as a base to make all strong acids appear equally strong, and it acts as an acid to make all strong bases appear equally strong
- oxyacid
- compound containing a nonmetal and one or more hydroxyl groups


