Hydrides
- Page ID
- 543
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The term hydride is commonly named after binary compounds that hydrogen forms with other elements of the periodic table. Hydride compounds in general form with almost any element, except a few noble gases. The trends and properties vary according to the type of intermolecular force that bonds the elements together, the temperature, its molecular masses, and other components. Hydrides are classified into three major groups, depending on what elements the hydrogen bonds to. The three major groups are covalent, ionic, and metallic hydrides. Formally, hydride is known as the negative ion of a hydrogen, H-, also called a hydride ion. Because of this negative charge, hydrides have reducing, or basic properties. Its special characteristics will be further discussed.
Covalent Hydrides
The first major group is covalent hydrides, which is when a hydrogen atom and one or more non-metals form compounds. This occurs when hydrogen covalently bonds to a more electropositive element by sharing electron pairs. These hydrides can be volatile or non-volatile. Volatile simply means being readily able to be vaporized at low temperatures. One such example of a covalent hydride is when hydrogen bonds with chlorine and forms hydrochloric acid (\(HCl\)). Examples are listed below:
\[\ce{H2(g) + Cl2(g) -> 2HCl(g)} \label{1} \]
\[\ce{3H2(g) + N2(g) -> 2NH3(g)} \label{2} \]
The hydrides of nonmetals on the periodic table become more electronegative as you move from group 13 to 17. This means that they are less capable of donating an electron, and want to keep them because their electron orbital becomes fuller. Instead of donating a \(H^-\), they would instead donate a \(H^+\) because they are more acidic.
Boron can form many different types of hydrides; one of them is borane (\(\ce{BH3}\)), which reacts violently with air and is easily oxidized. Borane occurs as a gaseous substance, and can form \(\ce{B2H6}\) by two borane molecules combined with each other. Borane is not a stable compound because it does not follow a complete octet rule since it has only six valence electrons.

Ammonia is an important nitrogen hydride that is possible due to the synthesis of nitrogen and water which is called the Haber-Bosch process. The chemical equation for this reaction is:
\[\ce{N2(g) + 3H2(g) <=> 2NH3(g)} \nonumber \]
To yield ammonia, there needs to be a catalyst to speed up the reaction, a high temperature and a high pressure. Ammonia is a reagent used in many chemistry experiments and is used as fertilizer. Ammonia can react with sulfuric acid to produce ammonium sulfate, which is also an important fertilizer. In this reaction, ammonia acts as a base since it receives electrons while sulfuric acid gives off electrons.
\[\ce{2NH3(aq) + H2SO4(aq) <=> (NH4)2SO4(aq)} \nonumber \]
Other hydrides of nitrogen include ammonium chloride, hydrazine and hydroxylamine. Ammonium chloride is widely used in dry-cell batteries and clean metals.
Ionic Hydrides
The second category of hydrides are ionic hydrides (also known as saline hydrides or pseudohalides). These compounds form between hydrogen and the most active metals, especially with the alkali and alkaline-earth metals of group one and two elements. In this group, the hydrogen acts as the hydride ion (\(H^-\)). They bond with more electropositive metal atoms. Ionic hydrides are usually binary compounds (i.e., only two elements in the compound) and are also insoluble in solutions.
\[\ce{A(s) + H2(g) -> 2AH(s)} \label{3} \]
with \(A\) as any group 1 metal.
\[\ce{A(s) + H2(g) -> AH2(s)} \label{4} \]
with \(A\) as any group 2 metal.
Ionic hydrides combine vigorously with water to produce hydrogen gas.
As ionic hydrides, alkali metal hydrides contain the hydride ion \(H^-\) as well. They are all very reactive and readily react with various compounds. For example, when an alkali metal reacts with hydrogen gas under heat, an ionic hydride is produced. Alkali metal hydrides also react with water to produce hydrogen gas and a hydroxide salt:
\[\ce{MH(s) + H2O(l) -> MOH(aq) + H2(g)} \nonumber \]
Metallic Hydrides
The third category of hydrides are metallic hydrides, also known as interstitial hydrides. Hydrogen bonds with transition metals. One interesting and unique characteristic of these hydrides are that they can be nonstoichiometric, meaning basically that the fraction of H atoms to the metals are not fixed. Nonstoichiometric compounds have a variable composition. The idea and basis for this is that with metal and hydrogen bonding there is a crystal lattice that H atoms can and may fill in between the lattice while some might, and is not a definite ordered filling. Thus it is not a fixed ratio of H atoms to the metals. Even so, metallic hydrides consist of more basic stoichiometric compounds as well.
Intermolecular Interactions
You may think that hydrides are all intact through hydrogen bonding because of the presence of at least a hydrogen atom, but that is false. Only some hydrides are connected with hydrogen bonding. Hydrogen bonds have energies of the order of 15-40 kJ/mol, which are fairly strong but in comparison with covalent bonds at energies greater than 150 kJ/mol, they are still much weaker. Some hydrogen bonding can be weak if they are mildly encountered with neighboring molecules. Specifically fluorine, oxygen, and nitrogen are more vulnerable to hydrogen bonding.
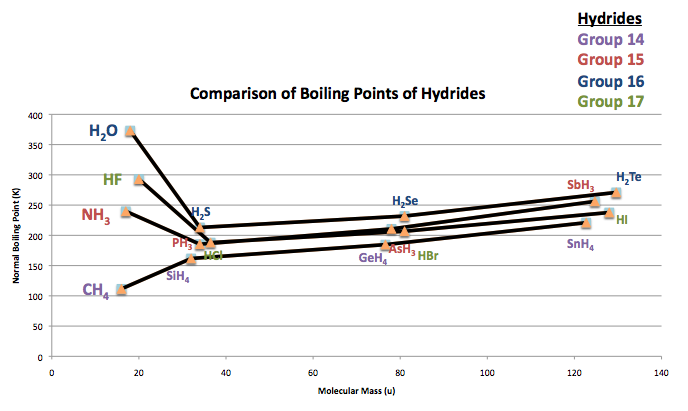
In hydrides, hydrogen is bonded with a highly electronegative atom so their properties are more distinguished. Such that in the chart below comparing boiling points of groups 14-17 hydrides, the values of ammonia (NH3), water (H2O), and hydrogen fluoride (HF) break the increasing boiling point trend.
Supposedly, as the molecular mass increases, the boiling points increase as well. Due to the hydrogen bonds of the three following hydrides, they distinctly have high boiling points instead of the initial assumption of having the lowest boiling points. What occur in these hydrogen bonds are strong dipole-dipole attractions because of the high ionic character of the compounds.
References
- General Chemistry:Principles and Modern Applications.-9th ed./Ralph H.Petrucci...
- General Chemistry.7th ed./Kenneth W. Whitten.
- Peruzzini, Maurizio, and Rinaldo Poli. Recent Advances in Hydride Chemistry. St. Louis: Elsevier, 2001.
- General Chemistry 9e. Boston: Houghton Mifflin, 2008.
Contributors
- Tandis Arani (UCD)

