Supercritical Fluids
- Page ID
- 1627
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Matter can be pushed to temperatures and pressures beyond those of its critical point. This stage is characterized by the inability to distinguish whether the matter is a liquid or a gas, as a result, Supercritical fluids (SCF) do not have a definite phase. In 1822 Baron Charles Cagniard de la Tour discovered supercritical fluids while conducting experiments involving the discontinuities of the sound of a flint ball in a sealed cannon barrel filled with various fluids at various temperatures ("Charles Cagniard de la Tour").
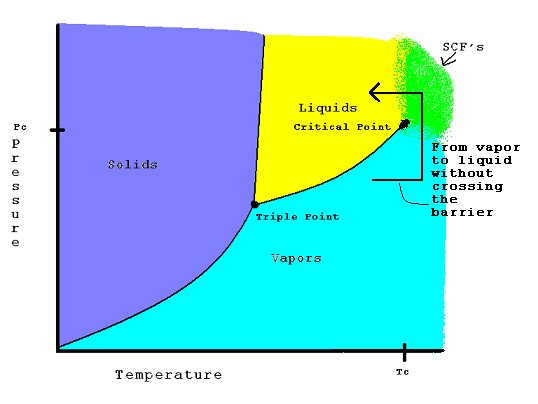
Supercritical fluids have the low viscosity of a gas and the high density of a liquid, making it impossible to liquefy the matter using any amount of pressure. However, it is possible to go from a gas to a liquid without crossing the boundary between the vapor and liquid phase using a supercritical fluid just by lowering the temperature of the liquid (Observe phase diagram below).
Volatile liquids and solids, or liquids and solids with a high vapor pressure or low boiling point, are soluble in gas. It becomes especially easy to dissolve liquids and solids such as these in a supercritical fluid because of the high density. As can be noted from the Mole Fraction Solubility. The Mole Fraction Solubility is simply the ratio of the sublimation (or vapor pressure) to the total gas pressure.
Supercritical fluids have no surface tension because they are not subject to the vapor-liquid boundary so no molecules have the attraction to the interior of the liquid. The densities and viscosity of a supercritical fluid are subject to change when pressure or temperature are tampered with, and the supercritical fluid of a substance can have very different properties than the regular fluids. For instance, water that is supercritical differs from regular water in the fact that it is non-polar and acidic (Benner 680).
Examples: A Phase Diagram:
Notice the yellow and blue mix to create green area that follows the Coordinates of the critical point, that is where the supercritical fluids occur on the graph. Each element and molecule have unique critical points. The arrow shows how it is possible to go from a vapor to a liquid by using supercritical fluids, pressure and temperature. Notice how when pressure and temperature on a gas are increased into the supercritical range, and the temperature is lowered, the substance moves into the liquid phase.
| Liquid | Critical Temperature (K) | Critical Pressure (atm) |
|---|---|---|
| Hydrogen (H) | 33.3 | 12.8 |
| Neon (Ne) | 44.4 | 26.3 |
| Nitrogen (N) | 126 | 33.5 |
| Argon (Ar) | 151 | 48.5 |
| Methane (CH4) | 191 | 45.8 |
| Ethane (C2H6) | 305 | 48.2 |
| Carbon Dioxide (CO2) | 305 | 72.9 |
| Ammonia (NH3) | 406 | 112 |
| Water (H2O) | 647 | 218 |
| (Benner 680) |
Natural Occurences
Supercritical fluids can occur in nature. For example, in places like underwater volcanoes, specifically those located deep beneath the ocean's surface, supercritical water is formed because of the immense pressure due to the depth and the intense heat from the vents of the volcano. This water can lead to the formation of crystals used in some jewelry (Benner 680).
The atmospheric pressure of Venus is approximately 90 times greater than that of the Earth, with an average temperature of 467 °C, and about 97% of its atmosphere is Carbon Dioxide. Therefore it would be reasonable to consider the atmosphere of Venus a supercritical fluid because both the pressure and temperature exceed that of Carbon Dioxide's critical point however this theory has not been proven. Examples similar to this one can be found throughout the solar system, particularly in the Gas Giants (Benner 680).
Supercritical fluids are useful in science today for purposes ranging from the extraction of floral fragrance from flowers and the process of creating decaffeinated coffee, to applications in food science and functional food ingredients, pharmaceuticals, cosmetics, polymers, powders, bio- and functional materials, nano-systems, natural products, biotechnology, fossil and biofuels, microelectronics and environment (Bottini 133).
Extraction using Supercritical Fluids is a fairly simple concept, and much more efficient than normal extraction methods, which require both heating and ventilation of the solution to the atmosphere. Supercritical fluids allow continuous extraction using common, inexpensive, and more importantly non-toxic materials, and only requires venting to separate the solvent from the material being removed. The extraction involves applying the supercritical solvent to whatever material is being eradicated, for example, coffee beans which are being decaffeinated, and allowing the solvent to remove the substance being extracted. Returning to the coffee bean example, supercritical CO2 would be applied to the beans, then when it had extracted the caffeine, the CO2 would be put through carbon filters, which would separate the caffeine from the solvent which can then be removed simply by venting because of its unique properties and similarities to vapor. Likewise, supercritical fluids can be used as solvents to apply substances like dyes to clothing, the process for this is more or less the reverse of extraction. The most commonly used solvents are supercritical Carbon Dioxide and Water because of their availability and low critical temperatures (Hardy).
References
- Benner, Steven, and Matthew Carrigan, Alonso Ricardo. Is There a Common Chemical Model for Life in the Universe?. Gainesville, FL: Elsevier Ltd., 2004.
- Bottini, Susana. "Preface." The Journal of Supercritical Fluids 45. 2.June 2008 133. 27 May 2008
- "Charles Canigard de la Tour." Wikipedia. Wikipedia. 29 May 2008
- Hardy, James K. "Supercritical Fluids." Chemical Separations (2005) 27 May 2008
- Petrucci, Ralph, and William Harwood. F. Geoffrey Herring. Jeffry Madura. General Chemistry: Principles and Modern Applications. 9th ed. Upper Saddle River, NJ: Pearson, 2007.
Contributors and Attributions
- Kiana Samadzadeh

