Liquid Crystals
- Page ID
- 3584
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)A true liquid is isotropic, meaning that its properties are uniform in all directions— the result of its molecules being in constant random motion. Crystalline solids, in contrast, are anisotropic; optical- and other properties such as thermal and electrical conductivity vary with direction. A liquid crystal phase has many of the physical attributes of a liquid, but its molecular units are sufficiently ordered to give rise to some anisotropy, most notably in their optical properties.
As with so many scientific discoveries, it all started with an unexpected observation. In 1888, an Austrian plant physiologist, working in Prague, attempted to measure the melting point of a cholesterol derivative that he had extracted from a plant. To his surprise, he found that this substance appeared to have two melting points. At 145° C the crystalline solid first melted into a cloudy liquid, and then at 178° the cloudiness suddenly disappeared, leaving the clear, transparent liquid that one ordinarily expects after melting.
A physicist who examined this material recognized that the cloudy liquid had a certain degree of order; he proposed that it was a hitherto unknown state of matter, and suggested the name "liquid crystal". But science was not quite ready to accept this concept, and despite a number of confirming experiments between 1910 and 1930, the field remained largely dormant until the mid-1960s when the French physicist Pierre-Gilles de Gennes (1932-2007) developed a thorough theoretical model for the properties of liquid crystals, particularly their ability to scatter light. For this and for related studies on polymers, de Gennes was awarded the 1991 Nobel Prize in Physics.
The structural units capable of forming liquid crystals are always molecules, usually rather elongated organic ones that possess dissimilar local structural regions that can interact in an organized way with their neighbors. Over a certain range of temperatures, these attractive forces can lead to a degree of self-organization in which crystal-like order persists in some directions even though it is lost in other directions. Although a large variety of molecules are known to form liquid crystals, the simplest and most common structures can be represented by the following generic scheme:
- The two benzene rings confer a degree of planarity on the molecule that promotes attractions between neighboring molecules. This planarity is enhanced when the linkage group contains a double bond such as -(HC=N)- which keeps the rings in the same plane.
- The terminal group is often (but not always) one that is somewhat polar, giving rise to intermolecular attractions along the long axis.
- The side chain is commonly a hydrocarbon chain that serves to elongate the molecule.
Properties of Liquid Crystals
Liquid crystal phases are generally cloudy in appearance, which means that they scatter light in much the same way as colloids such as milk. This light scattering is a consequence of fluctuating regions of non-uniformity as small groups of molecules form and disperse.

The anisotropy of liquid crystals causes them to exhibit birefringence. That is, light that enters the crystal is broken up into two oppositely-polarized rays that travel at different velocities. Observation of a birefringent material between crossed polarizing filters reveals striking patterns and color effects.

The colors arise from interference between the ordinary ray and the extraordinary ray; the latter traverses a slightly longer path through the material, and thus emerges later (and out-of-phase) with the former.
Liquid crystals, like all other kinds of matter, are subject to thermal expansion. As the temperature rises, the average spacing between the aligned molecules of a nematic phase (see below) increases, thus causing the e-ray to be increasingly retarded with respect to the o-ray. If a suitable liquid crystal mixture is painted onto the surface of a patient's body, it can often reveal the sites of infection or tumors, which cause increases or reductions in local blood flow giving rise to temperature anomalies. Inexpensive thermometers can be made by printing a succession of suitably formulated LC mixtures on a paper or plastic strip which is held in contact with the surface whose temperature is to be measured.
The first working liquid crystal display ("LCD") was demonstrated at RCA in 1968. In the following year, James Fergason of Kent State University (OH) discovered the twisted nematic field effect which allowed a much higher-quality display and led to the first commercial LCD wristwatch in 1979. LCDs were first used in calculators in the late 1970s, but they are now widely encountered in computer- and television displays. The thickness of the chiral phase is such that polarized light passing through it is rotated by 90°, which corresponds to the orientation of the right-hand polarizing filter. So in this state, the light passes through and illuminates the pixel surface.
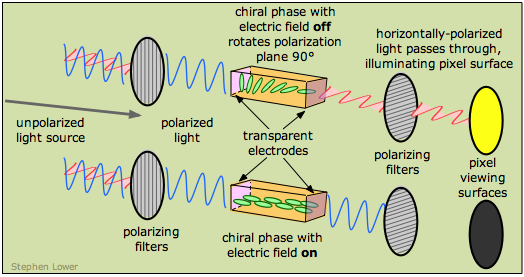
When an electric field is imposed on the liquid crystal phase, the component molecules line up in the field and the chirality is lost. Light passing through the cell does not undergo rotation of its polarization plane, and is therefore stopped by the right polarizing filter, turning the display off.
Nematic and Smectic Phases
There are many classes and sub-classes of liquid crystals, but for the purposes we will divide them into the two kinds depicted on the right side of this Figure below which also compares them with the two conventional condensed phases of matter:
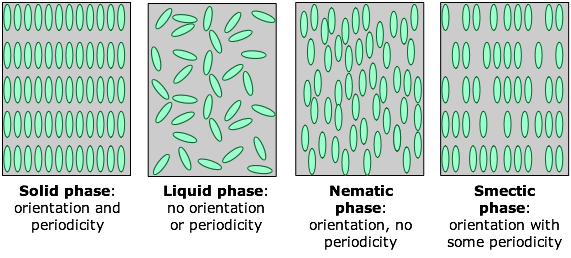
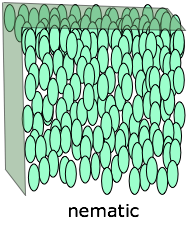
In a nematic phase (the term means "thread-like") the molecules are aligned in the same direction but are free to drift around randomly, very much as in an ordinary liquid. Owing to their polarity, the alignment of the rod-like molecules can be controlled by applying an electric field; this is the physical basis for liquid crystal displays and certain other electrooptic devices.
In smectic ("soap-like") phases the molecules are arranged in layers, with the long molecular axes approximately perpendicular to the laminar planes. The only long-range order extends along this axis, with the result that individual layers can slip over each other (hence the "soap-like" nature) in a manner similar to that observed in graphite. Within a layer there is a certain amount of short-range order. There are a large number of sub-categories of smectic phases which we will not go into here.

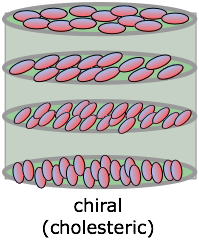
Chiral phases
Special cases of nematic and smectic phases are sometimes formed by molecules that display chirality — that is, they can exist in either left- or right-handed forms that cannot be superposed on each other. In the resulting chiral phase, successive molecules positioned along the long axis are rotated around this axis, giving rise to a periodicity that repeats itself at distances corresponding to a complete rotation. These twisted phases are able to rotate the plane of polarized light that passes along the axis. If the molecules are polar, this twisting can be turned off by imposing an external electric field at either end of the long axis. Besides the very important application of this property (known as ferroelectricity) to liquid crystal displays, these materials can be used to make electrooptic shutters which can be switched open and closed in microseconds.
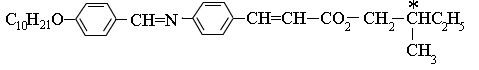
A typical chiral molecule capable of exhibiting ferroelectric behavior is shown below. The chiral part of the molecule is indicated by the asterisk. The chirality arises because this carbon atom is joined to four different groups.
Contributors and Attributions
Stephen Lower, Professor Emeritus (Simon Fraser U.) Chem1 Virtual Textbook
Thumnbnail: Schematic of mesogen ordering in the smectic liquid crystal phases: smectic-A (layered) and smectic-C (layered and tilted). (CC -SA-BY 3.0; Kebes).


