The Hydronium Ion
- Page ID
- 1290
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Owing to the overwhelming excess of \(\ce{H2O}\) molecules in aqueous solutions, a bare hydrogen ion has no chance of surviving in water.
Free Hydrogen Ions do not Exist in Water
The hydrogen ion in aqueous solution is no more than a proton, a bare nucleus. Although it carries only a single unit of positive charge, this charge is concentrated into a volume of space that is only about a hundred-millionth as large as the volume occupied by the smallest atom. (Think of a pebble sitting in the middle of a sports stadium!) The resulting extraordinarily high charge density of the proton strongly attracts it to any part of a nearby atom or molecule in which there is an excess of negative charge. In the case of water, this will be the lone pair (unshared) electrons of the oxygen atom; the tiny proton will be buried within the lone pair and will form a shared-electron (coordinate) bond with it, creating a hydronium ion, \(\ce{H3O^{+}}\). In a sense, \(\ce{H2O}\) is acting as a base here, and the product \(\ce{H3O^{+}}\) is the conjugate acid of water:
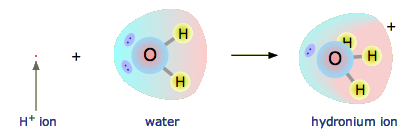
Although other kinds of dissolved ions have water molecules bound to them more or less tightly, the interaction between H+ and \(\ce{H2O}\) is so strong that writing “\(\ce{H^{+}(aq)}\)” hardly does it justice, although it is formally correct. The formula \(\ce{H3O^{+}}\) more adequately conveys the sense that it is both a molecule in its own right, and is also the conjugate acid of water.
The equation
\[\ce{HA → H^{+} + A^{–}} \nonumber\]
is so much easier to write that chemists still use it to represent acid-base reactions in contexts in which the proton donor-acceptor mechanism does not need to be emphasized. Thus, it is permissible to talk about “hydrogen ions” and use the formula \(\ce{H^{+}}\) in writing chemical equations as long as you remember that they are not to be taken literally in the context of aqueous solutions.
Interestingly, experiments indicate that the proton does not stick to a single \(\ce{H2O}\) molecule, but changes partners many times per second. This molecular promiscuity, a consequence of the uniquely small size and mass the proton, allows it to move through the solution by rapidly hopping from one \(\ce{H2O}\) molecule to the next, creating a new \(\ce{H3O^{+}}\) ion as it goes. The overall effect is the same as if the \(\ce{H3O^{+}}\) ion itself were moving. Similarly, a hydroxide ion, which can be considered to be a “proton hole” in the water, serves as a landing point for a proton from another \(\ce{H2O}\) molecule, so that the OH– ion hops about in the same way.
Because hydronium and hydroxide ions can “move without actually moving” and thus without having to plow their way through the solution by shoving aside water molecules, as do other ions, solutions which are acidic or alkaline have extraordinarily high electrical conductivities.
Reaction
The hydronium ion is an important factor when dealing with chemical reactions that occur in aqueous solutions. Its concentration relative to hydroxide is a direct measure of the pH of a solution. It can be formed when an acid is present in water or simply in pure water. It's chemical formula is \(\ce{H3O^{+}}\). It can also be formed by the combination of a H+ ion with an \(\ce{H2O}\) molecule. The hydronium ion has a trigonal pyramidal geometry and is composed of three hydrogen atoms and one oxygen atom. There is a lone pair of electrons on the oxygen giving it this shape. The bond angle between the atoms is 113 degrees.
\[H_2O_{(l)} \rightleftharpoons OH^-_{(aq)} + H^+_{(aq)}\]
As H+ ions are formed, they bond with \(\ce{H2O}\) molecules in the solution to form \(\ce{H3O^{+}}\) (the hydronium ion). This is because hydrogen ions do not exist in aqueous solutions, but take the form of the hydronium ion, \(\ce{H3O^{+}}\). A reversible reaction is one in which the reaction goes both ways. In other words, the water molecules dissociate while the OH- ions combine with the H+ ions to form water. Water has the ability to attract H+ ions because it is a polar molecule. This means that it has a partial charge, in this case the charge is negative. The partial charge is caused by the fact that oxygen is more electronegative than hydrogen. This means that in the bond between hydrogen and oxygen, oxygen "pulls" harder on the shared electrons thus causing a partial negative charge on the molecule and causing it to be attracted to the positive charge of H+ to form hydronium. Another way to describe why the water molecule is considered polar is through the concept of dipole moment. The electron geometry of water is tetrahedral and the molecular geometry is bent. This bent geometry is asymmetrical, which causes the molecule to be polar and have a dipole moment, resulting in a partial charge.
An overall reaction for the dissociation of water to form hydronium can be seen here:
\[2 H_2O_{(l)} \rightleftharpoons OH^-_{(aq)} + H_3O^+_{(aq)}\]
With Acids
Hydronium not only forms as a result of the dissociation of water, but also forms when water is in the presence of an acid. As the acid dissociates, the H+ ions bond with water molecules to form hydronium, as seen here when hydrochloric acid is in the presence of water:
\[HCl (aq) + H_2O \rightarrow H_3O^+ (aq) + Cl^-(aq)\]
pH
The pH of a solution depends on its hydronium concentration. In a sample of pure water, the hydronium concentration is \(1 \times 10^{-7}\) moles per liter (0.0000001 M) at room temperature. The equation to find the pH of a solution using its hydronium concentration is:
\[pH = -\log {(H_3O^+)}\]
Using this equation, we find the pH of pure water to be 7. This is considered to be neutral on the pH scale. The pH can either go up or down depending on the change in hydronium concentration. If the hydronium concentration increases, the pH decreases, causing the solution to become more acidic. This happens when an acid is introduced. As H+ ions dissociate from the acid and bond with water, they form hydronium ions, thus increasing the hydronium concentration of the solution. If the hydronium concentration decreases, the pH increases, resulting in a solution that is less acidic and more basic. This is caused by the \(OH^-\) ions that dissociate from bases. These ions bond with H+ ions from the dissociation of water to form \(\ce{H2O}\) rather than hydronium ions.
A variation of the equation can be used to calculate the hydronium concentration when a pH is given to us:
\[H_3O^+ = 10^{-pH} \]
When the pH of 7 is plugged into this equation, we get a concentration of 0.0000001 M as we should.
Learning to use mathematical formulas to calculate the acidity and basicity of solutions can be difficult. Here is a video tutorial on the subject of calculating hydronium ion concentrations:
Configuration of Hydronium in Water
It is believed that, on average, every hydronium ion is attracted to six water molecules that are not attracted to any other hydronium ions. This topic is still currently under debate and no real answer has been found.
Problems
- Determine the pH of a solution that has a hydronium concentration of 2.6x10-4M.
- Determine the hydronium concentration of a solution that has a pH of 1.7.
- If a solution has a hydronium concentration of 3.6x10-8M would this solution be basic or acidic?
- What is the pH of a solution that has 12.2 grams of hydrochloric acid in 500 ml of water?
- Why do acids cause burns?
Answers
1. Remembering the equation: pH = -log[H3O]
Plug in what is given: \(pH = -\log[2.6 \times 10^{-4}\;M]\)
When entered into a calculator: pH = 3.6
2. Remembering the equation: [H3O] = 10-pH
Plug in what is given: [H3O] = 10-1.7
When entered into a calculator: 1.995x10-2M
3. Determine pH the same way we did in question one: pH = -log[3.6x10-8]
pH = 7.4
Because this pH is above 7 it is considered to be basic.
4. First write out the balanced equation of the reaction: \[HCl_{(aq)} + H_2O_{(l)} \rightleftharpoons H_3O^+_{(aq)} + Cl^-_{(aq)}\]
Notice that the amount of HCl is equal to the amount of \(\ce{H3O^{+}}\) produced due to the fact that all of the stoichiometric coefficents are one.
So if we can figure out concentration of HCl we can figure out concentration of hydronium.
Notice that the amount of HCl given to us is provided in grams. This needs to be changed to moles in order to find concentration:
\[12.2\;g\; HCl \times \dfrac{1 \;mol\; HCl}{36.457\; g} = 0.335\; mol\; HCl\]
Concentration is defined as moles per liter so we convert the 500mL of water to liters and get .5 liters.
\[\dfrac{0.335\; mol\; HCl}{0.5\; L} = 0.67\;M\]
Using this concentration we can obtain pH: pH = -log[.67M]
\[pH = 0.17\]
5. Acids cause burns because they dehydrate the cells they are exposed to. This is caused by the dissociation that occurs in acids where H+ ions are formed. These H+ ions bond with water in the cell and thus dehydrate them to cause cell damage and burns.
References
- Petrucci, Harwood, Herring, Madura. General Chemistry Principles & Modern Applications. Prentice Hall. New Jersey, 2007.
- Marx, Tuckerman, Hutter, J. & Parrinello, M. (1999) The nature of the hydrated excess proton in water
- Petrucci, Ralph H. General Chemistry: Principles and Modern Applications. Upper Saddle River, NJ: Pearson/Prentice Hall, 2007. Print.


