Leaving Groups
- Page ID
- 1147
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Four Factors to Consider in Determining the Relative Ease at Which SN2 Displacement Occurs
- The nature of the leaving group (SN2 Reactions-The Leaving Group)
- The reactivity of the nucleophile (SN2 Reactions-The Nucleophile)
- The solvent (SN2 Reactions-The Nucleophile)
- The structure of the alkyl portion of the substrate (SN2 Reactions-The Substrate)
The Nature of the Leaving Group
In order to understand the nature of the leaving group, it is important to first discuss factors that help determine whether a species will be a strong base or weak base. If you remember from general chemistry, a Lewis base is defined as a species that donates a pair of electrons to form a covalent bond. The factors that will determine whether a species wants to share its electrons or not include electronegativity, size, and resonance.
As Electronegativity Increases, Basicity Decreases: In general, if we move from the left of the periodic table to the right of the periodic table as shown in the diagram below, electronegativity increases. As electronegativity increases, basicity will decrease, meaning a species will be less likely to act as base; that is, the species will be less likely to share its electrons.

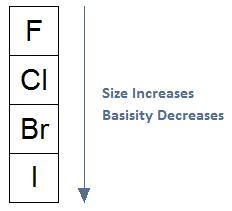
As Size Increases, Basicity Decreases: In general, if we move from the top of the periodic table to the bottom of the periodic table as shown in the diagram below, the size of an atom will increase. As size increases, basicity will decrease, meaning a species will be less likely to act as a base; that is, the species will be less likely to share its electrons.
Resonance Decreases Basicity: The third factor to consider in determining whether or not a species will be a strong or weak base is resonance. As you may remember from general chemistry, the formation of a resonance stabilized structure results in a species that is less willing to share its electrons. Since strong bases, by definition, want to share their electrons, resonance stabilized structures are weak bases.
Weak Bases are the Best Leaving Groups
Now that we understand how electronegativity, size, and resonance affect basicity, we can combine these concepts with the fact that weak bases make the best leaving groups. Think about why this might be true. In order for a leaving group to leave, it must be able to accept electrons. A strong bases wants to donate electrons; therefore, the leaving group must be a weak base. We will now revisit electronegativity, size, and resonance, moving our focus to the leaving group, as well providing actual examples.
As Electronegativity Increases, The Ability of the Leaving Group to Leave Increases.
As mentioned previously, if we move from left to right on the periodic table, electronegativity increases. With an increase in electronegativity, basisity decreases, and the ability of the leaving group to leave increases. This is because an increase in electronegativity results in a species that wants to hold onto its electrons rather than donate them. The following diagram illustrates this concept, showing -CH3 to be the worst leaving group and F- to be the best leaving group. This particular example should only be used to facilitate your understanding of this concept. In real reaction mechanisms, these groups are not good leaving groups at all. For example, fluoride is such a poor leaving group that SN2 reactions of fluoroalkanes are rarely observed.
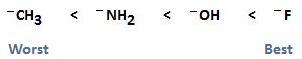
As Size Increases, The Ability of the Leaving Group to Leave Increases: Here we revisit the effect size has on basicity. If we move down the periodic table, size increases. With an increase in size, basicity decreases, and the ability of the leaving group to leave increases. The relationship among the following halogens, unlike the previous example, is true to what we will see in upcoming reaction mechanisms.

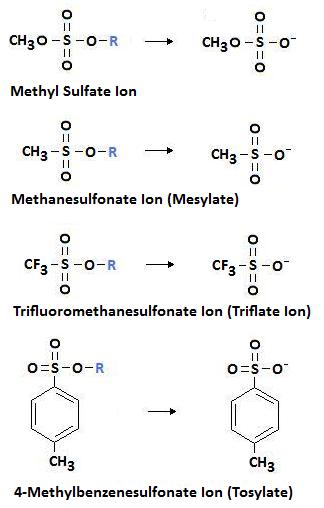
Resonance Increases the Ability of the Leaving Group to Leave: As we learned previously, resonance stabilized structures are weak bases. Therefore, leaving groups that form resonance structures upon leaving are considered to be excellent leaving groups. The following diagram shows sulfur derivatives of the type ROSO3- and RSO3-. Alkyl sulfates and sulfonates like the ones shown make excellent leaving groups. This is due to the formation of a resonance stabilized structure upon leaving.