6: Alcohols and an introduction to thiols, amines, ethers and sulfides
- Page ID
- 353906
In this chapter, we are going to take a closer look at the families of compounds that have carbon linked through a single covalent bond to an \(\mathrm{O}\), \(\mathrm{N}\), or \(\mathrm{S}\). These are known as alcohols (\(\mathrm{R-OH}\)), amines (\(\mathrm{R-NH}_{2}\), \(\mathrm{RR}^{\prime}\mathrm{-NH}\), \(\mathrm{RR}^{\prime}\mathrm{R}^{\prime \prime}\mathrm{-N}\)), thiols (\(\mathrm{R-SH}\)), ethers (\(\mathrm{R-OR}^{\prime}\)), and sulfides (\(\mathrm{R-SR}^{\prime}\)). We group these compounds together based on the predictable similarities and differences in their chemical and physical properties, specifically the fact that each of these functional groups has a relatively electronegative element (\(\mathrm{O}\), \(\mathrm{N}\) or \(\mathrm{S}\)) attached by a single bond to carbon and each has available lone electron pairs that can be donated to H+ or other electrophiles. The result is that alcohols, thiols, and amines (primary and secondary) all have relatively acidic hydrogens, which influences their chemical reactivities, and all show nucleophilic properties.
Table \(6.0.1\) Examples of Functional groups, their names and approximate \(\mathrm{pK}_{\mathrm{a}}\)‘s
| Functional Group | Example | Name | \(\mathrm{pK}_{\mathrm{a}}\) |
| Alcohol | 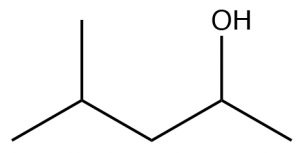 |
Remove -ane, add -ol.
4-methylpentan-2-ol |
(approximate)
\(\sim 15-16\) |
| Alcohol | 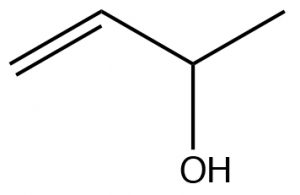 |
Alcohols take precedence over
alkenes, But-3-en-2-ol |
|
| Thiol | 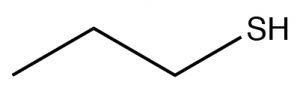 |
Longest chain, add -thiol
Propane-1-thiol |
\(\sim 10\) |
| Primary amine | 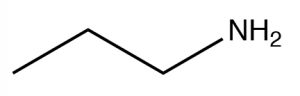 |
Longest chain, remove e, add
-amine Propanamine or Propyl amine |
\(\sim 33\) |
| Secondary amine | 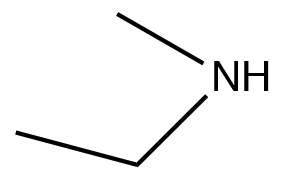 |
N-methylethanamine | \(\sim 33\) |
| Tertiary amine | 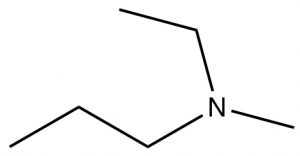 |
N-ethyl-N-methylpropanamine | N/A |
| Ether | 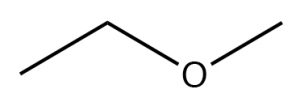 |
Methoxyethane
Ethyl methyl ether |
N/A |
| Sulfide | 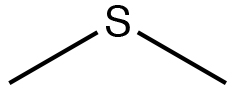 |
Dimethylsulfane
Dimethyl sulfide |
N/A |
We will concentrate our discussion on oxygenated compounds, but we will note reactivities across the various groups to illustrate their similarities (and differences).


