6.6: Preparation of Alcohols
- Page ID
- 354431
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)We have already seen several methods by which alcohols can be produced, mostly in Chapter \(5\). For example, the addition of water across a double bond, either through acid catalysis (Markovnikov addition) or by hydroboration/oxidation (Anti-Markovnikov addition), produces alcohols. We have also seen, under certain conditions, that alcohols can be produced by nucleophilic substitution. Both \(\mathrm{S}_{\mathrm{N}} 1\) and \(\mathrm{S}_{\mathrm{N}} 2\) reactions can produce alcohols, and now would be a good time to review all of these reactions (covered in Chapters \(1\), \(3\), \(4\) and \(5\)).
A reaction that we have not yet encountered is the reduction of carbonyl compounds. For example, a ketone such as acetone can be reduced through a reaction with sodium borohydride (\(\mathrm{NaBH}_{4}\)) or lithium aluminum hydride (\(\mathrm{LiAlH}_{4}\))[7]; both of these molecules can deliver hydride (\(\mathrm{H}^{-}\)) to the partially positive carbon of the carbonyl.
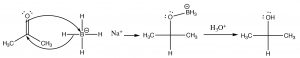
Sodium borohydride (\(\mathrm{NaBH}_{4}\)) is generally the reagent of choice as it is less reactive and the reaction can be carried in an open flask, whereas \(\mathrm{LiAlH}_{4}\) typically must be used with solvents that do not contain water and under a dry atmosphere. The intermediate \(\mathrm{R-O-BH}_{3}\) complex is destroyed by adding aqueous acid to give the final alcohol product.
Reactions where hydride is delivered to a carbonyl are similar to a reaction found in biological systems. \(\mathrm{NADH}\) (Nicotinamide Adenine Dinucleotide Hydride) is an unstable intermediate generated through a number of metabolic processes (such as fermentation), while not as reactive as \(\mathrm{NaBH}_{4}\), and (like, essentially, all biological reactions) requires a catalyst (an enzyme) to bring about the reduction of carbonyls; but the mechanism is similar. The reaction (like all reactions) is also reversible, so that the oxidized version of \(\mathrm{NADH}\), \(\mathrm{NAD}^{+}\), can accept a hydride from an alcohol to produce a ketone. In the mechanism (with only the nicotinamide part of \(\mathrm{NADH}\) shown) the “\(\mathrm{R}\)” group attached to the \(\mathrm{N}\) in the ring is actually a complex molecule consisting of an adenine moiety (a base found in nucleic acids and nucleotides), two sugar units (ribose), and two phosphate linkages. For now, let us focus on the similarities between the reduction reactions discussed above and those that take place in biological systems.
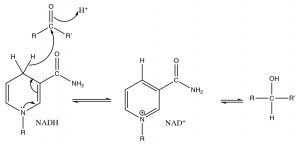
Reduction of a carbonyl by \(\mathrm{NADH}\) by delivery of \(\mathrm{H}^{-}\) to the carbonyl carbon
The conversion of pyruvic acid to lactic acid during glycolysis is just such an example. By looking at simpler systems, we can understand (and model) the types of reactions that occur in organisms.
Alcohols can also be produced by direct reduction with \(\mathrm{H}_{2}\)(g) using a transition metal catalyst, in a way similar to the reduction of \(\mathrm{C=C}\), except that the hydrogens add across the \(\mathrm{C=O}\).
The choice of reducing agent depends on presence of other functional groups within the molecule. For example, if we wanted to reduce a carbonyl group in a molecule that also had a carbon-carbon double bond, we would not use \(\mathrm{H}_{2} / \mathrm{Pd}\) as the reagent/catalyst, since it would also reduce the double bond as shown here (\(\rightarrow\)).
Preparation of alcohols with Grignard reagents:
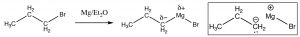
Just as we can add hydride ion by a nucleophilic attack at a carbonyl, we can also add an alkyl group, which formally contains a carbanion (a negatively charged carbon). The most common way to do this is via a Grignard[8] reagent, produced by reacting an alkyl halide with magnesium metal in a dry atmosphere with a non-protic solvent such as diethyl ether (\(\mathrm{Et}_{2}\mathrm{O}\)). The resulting Grignard reagent, \(\mathrm{RMgBr}\), is now polarized with a large partial negative charge on the carbon.
For our purposes, we can treat the Grignard as if it were a carbanion, which will react with a carbonyl group in much the same way as a hydride ion.[9]
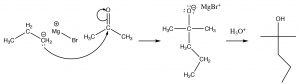
Reaction of a Grignard reagent with a ketone to give an alcohol
This reaction is applicable to a wide range of compounds that contain carbonyl groups including aldehydes and ketones, and (as we will see later) to esters and acid chlorides, but not carboxylic acids (why do you think that is?).


