6.1: (Brønsted) Acidity of Alcohols, Thiols, and Amines
- Page ID
- 354415
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Recall that there are several factors that can influence Brønsted acidity. These include: the strength of the bond between the \(\mathrm{R}\) (\(\mathrm{C}\)) and the \(\mathrm{O}\), \(\mathrm{S}\), or \(\mathrm{N}\) (denoted by “\(\mathrm{Y}\)” below) and \(\mathrm{H}\); the polarity of the bond; and the stability of the resulting anion (“\(\mathrm{Y}^{-}\)“). \[\mathrm{R}-\mathrm{Y}-\mathrm{H}+\mathrm{B}^{-} \rightarrow \mathrm{BH}+\mathrm{R}-\mathrm{Y}^{-}\]
Simple alcohols have acidities that are about the same as water, with a \(\mathrm{pK}_{\mathrm{a}}\) of around \(15-16\) (remember the \(\mathrm{pK}_{\mathrm{a}}\) of water is \(14\)). In comparison, amines are much less acidic, with \(\mathrm{pK}_{\mathrm{a}}\)‘s around \(33-36\). We understand this difference based on the fact that \(\mathrm{O}\) is more electronegative than \(\mathrm{N}\), therefore the \(\mathrm{O-H}\) bond is more polarized than the \(\mathrm{N-H}\) bond—making the partial positive charge on the \(\mathrm{H}\) larger in \(\mathrm{O-H}\) than in \(\mathrm{N-H}\) bonds. In polar solvents, this means that the \(\mathrm{H}\) bonded to an \(\mathrm{O}\) is better solvated and the proton is easier to remove than an \(\mathrm{H}\) bonded to an \(\mathrm{N}\). Similarly, the resulting negative charge on the anion is more stable on \(\mathrm{O}\) than on \(\mathrm{N}\) because the effective nuclear charge on \(\mathrm{O}\) is greater (which is the cause of its greater electronegativity, compared with \(\mathrm{N}\)). In contrast, thiols are more acidic than alcohols because the \(\mathrm{S-H}\) bond is weaker—the size of \(\mathrm{S}\) and \(\mathrm{H}\) orbitals results in smaller overlap and therefore weaker bonds (just like \(\mathrm{HBr}\) is more acidic than \(\mathrm{HCl}\)) and the resulting anion is more stable because the larger size of \(\mathrm{S}\) results in the negative charge being distributed over a larger volume.
To deprotonate a simple alcohol, with a \(\mathrm{pK}_{\mathrm{a}}\) of around \(15\), requires a base that forms a bond with \(\mathrm{H}\) that is than the bond that would be formed with the resultant alkoxide (\(\mathrm{RO}^{-}\))—otherwise the acid-base reaction would reverse. For this reason, we cannot use a base like sodium hydroxide, because its conjugate acid \(\mathrm{H}_{2} \mathrm{O}\) has a \(\mathrm{pK}_{\mathrm{a}}\) of around \(14\). Therefore, although some alcohol deprotonation would occur in water, the equilibrium position would be somewhere in the middle, so that both \({}^{-}\mathrm{OH}\) and \(\mathrm{RO}^{-}\) would be present in the reaction mixture. To completely deprotonate an alcohol with a \(\mathrm{pK}_{\mathrm{a}}\) of around \(15\), we would need to use a stronger base, such as sodium hydride[1] (\(\mathrm{NaH}\)), or sodium amide[2] (\(\mathrm{NaNH}_{2}\)).
![]()
Note that the reaction must take place in an aprotic solvent; in this case diethyl ether is used (otherwise the \(\mathrm{NaH}\) would react with the solvent as well as the alcohol). Another common method to deprotonate alcohols is through a redox reaction, using a group one metal—usually sodium.
![]()
Some alcohols are much more acidic; for example \(-\mathrm{OH}\) groups attached to an aromatic ring (which are called phenols) typically have \(\mathrm{pK}_{\mathrm{a}}\)‘s around \(10\). This difference in \(\mathrm{pK}_{\mathrm{a}}\) must lie with the nature of the conjugate base (the anion), since the same \(\mathrm{O-H}\) bond breaks during the proton transfer. In this case, the conjugate base is stabilized by delocalizing the electrons to the aromatic ring by resonance. Recall that the more delocalized a charge (in this case the electrons), the more stable the resultant ion is.
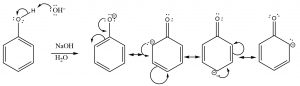
Phenols are more acidic than typical alcohols because the conjugate base is stabilized by resonance
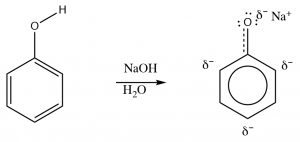
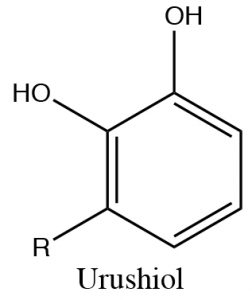
Phenols can be deprotonated by \(\mathrm{NaOH}\) because the phenolate anion is more stable than hydroxide. Therefore, phenols are soluble in aqueous solutions of sodium hydroxide. This also provides a way of separating phenols from other (non-acidic) organic substances, since the phenol can be regenerated simply by adding acid. One practical way that this phenomenon can be used is to remove the highly allergenic substances secreted by poison ivy (or oak or sumac) plants. The major allergen belongs to a family of di-phenols called urushiol. The \(\mathrm{R}\) can by any of a number of long chained hydrocarbons. Washing the affected part with a basic solution (soap for example) will help solubilize the urushiol and remove it from the skin.
Alcohol acidity can also be increased by inductive electron withdrawal (due to the presence of electronegative atoms linked through sigma bonds) just as we discussed earlier in the case of carboxylic acids: for example \(\mathrm{CF}_{3} \mathrm{OH}\) is more acidic than \(\mathrm{CH}_{3} \mathrm{OH}\).
We might also predict the effects relative to acidities of amines and thiols in terms of resonance and inductive stabilization, but, in fact, most of their chemistry is not associated with acidity and we will not dwell on this idea here.


