5: Alkenes and Alkynes
- Page ID
- 353905
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)When a carbon is bonded to one or more electronegative atoms, it takes on a partial positive charge and it is electrophilic. Such electrophilic carbons can undergo nucleophilicsubstitution or elimination reactions, or both, depending upon the structures of the reacting molecules, the strength of the nucleophile, and the type of solvent in which the reaction occurs. Now, we turn to reactions that electron-rich carbon species can undergo.
Alkenes and alkynes.
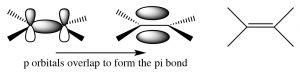
Both alkenes and alkynes are “unsaturated,” which means that they contain double or triple carbon-carbon bonds. The term unsaturated comes from the fact that more \(\mathrm{H}\) atoms can be added to these molecules across the double or triple bonds. A simple alkene contains a pair of carbons linked by a double bond; this double bond consists of a sigma bond and a pi bond. The sigma bond is formed by end-to-end overlap of \(\mathrm{sp}^{2}\) hybrid orbitals, and the pi bond by side-to-side overlap of the p orbitals. A pi bond has two lobes of electron density above and below the plane of the molecule. There are a number of consequences to this arrangement:
- the resulting region of the molecule is planar (the molecule is said to have trigonal planar geometry),
- the electron density between the two carbons is high because there are four electrons in this region instead of two, and
- rotation around a double bond is constrained (in contrast to rotation around a single bond).
Rotation around a double bond requires breaking the overlap of the pi bond and its subsequent reformation. As with all bond-breaking phenomena, the bond-breaking step requires energy; in fact, significantly more energy than is required to bring about rotation around a single bond where no bond-breaking occurs. As we will see, these three factors have a marked effect on the behavior of alkenes.
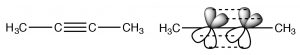
Alkynes are compounds that contain triple bonds. The triple bond consists of one sigma bond formed from end-to-end overlap of sp-hybrid orbitals and two pi bonds formed from side to side overlap. The carbons are \(\mathrm{sp}\)-hybridized and the molecule is linear in the region of the triple bond; again rotation around a triple bond is constrained—two pi bonds must be broken for it to occur (which requires an input of energy). This bonding arrangement results in a very electron rich \(\mathrm{C-C}\) region with the sigma bond inside what looks like a cylinder of pi electron density.
Naming Alkenes
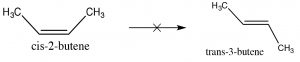
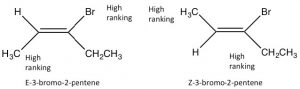
Since alkenes have restricted rotation around the \(\mathrm{C=C}\) group, they can exist as stereoisomers. For example, in 2-butene there is a methyl and an \(\mathrm{H}\) bonded to each of the double-bonded carbons (carbons 2 and 3 of the molecule). Because the \(\mathrm{C=C}\) group is planar, the \(\mathrm{CH}_{3}\) groups can be on either the same (“cis”) or opposite (“trans”) sides of the double bond (\(\rightarrow\)); this cis/trans nomenclature is similar to that we used with cyclohexane rings. As the groups attached to each carbon get more complex, such nomenclature quickly becomes confusing. To cope, we turn to another established naming scheme; in this case, the Cahn-Ingold-Prelog convention we previously used with chiral centers. This involves ranking the groups linked to each double-bond carbon. If the high groups are together (same side), the name is prefixed by Z (from the German word for together: zusammen). If they are on opposite sides, they are labeled E (entgegen; away). E and Z isomers are diastereoisomers: they have the same connectivity but neither can be superimposed on its mirror image. In E-3-bromo-2-pentene, the \(\mathrm{CH}_{3}\) and \(\mathrm{CH}_{2} \mathrm{CH}_{3}\) groups are closer to one another than they are in Z-3-bromo-2-pentene; the result is that they have different physical and chemical properties. These differences make it possible to separate E and Z isomers (and cis/trans since they are just a special case of E/Z) from one another.
Stability of alkenes:
Elimination reactions that produce alkenes tend to favor the most substituted alkene as the major product. The relative stabilities of various alkenes can be determined by reacting the alkene with hydrogen and determining the enthalpy change (\(\Delta \mathrm{H}\)).
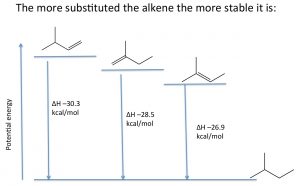
For example, shown (\(\rightarrow\)), the three different alkenes produce the same product, and therefore the differences in the energy released must arise from the fact that the initial alkenes have different energies. The more alkyl groups attached to the double bond, the more stable (less reactive) the alkene is, and therefore a lower amount of energy is released. Molecular stability in alkenes is attributed to the same causes as the relative stabilities of carbocations; alkyl groups stabilize the pi bond by hyperconjugation and induction.


