5.3: Reduction of Alkenes-
- Page ID
- 354411
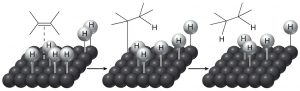
The historical meaning of “reduction” involved reactions with hydrogen (\(\mathrm{H}_{2}\)), and conversely, oxidation meant reaction with oxygen (\(\mathrm{O}_{2}\)). This makes sense from the perspective that carbon is slightly more electronegative than hydrogen, so that a \(\mathrm{C-H}\) bond is polarized as \(\mathrm{C}^{\partial-}\) and \(\mathrm{H}^{\partial+}\). Therefore, adding hydrogen to a \(\mathrm{C=H}\) will increase (slightly) the negative charge on the carbon. (Similarly, a \(\mathrm{C-O}\) bond is polarized \(\mathrm{C}^{\partial+}\) and \(\mathrm{O}^{\partial-}\), so that adding more oxygens to a carbon increases the amount of positive charge on the carbon.) Even today we refer to adding hydrogen across pi bonds as a reduction. However, alkenes do not normally react with hydrogen; typically a catalyst (usually a transition metal) is necessary for the reaction to occur. In general, the catalyst is supplied as a finely divided powder adsorbed onto an inert substance such as charcoal. The, most common catalysts are platinum or palladium on charcoal (\(\mathrm{Pt} / \mathrm{C}\) or \(\mathrm{Pt} / \mathrm{C}\)). Typically, the substance to be reduced is dissolved in a solvent, the catalyst is added, and then hydrogen is bubbled through the mixture. The catalyst adsorbs both \(\mathrm{H}_{2}\) and the alkene onto its surface and this interaction weakens both the \(\mathrm{H}_{2}\) bond and the pi bond. The hydrogen then migrates to the adsorbed alkene and adds across the double bond. The reaction is stereospecific in that both H’s add from the same side—a syn addition. This can be seen more clearly if we use deuterium instead of hydrogen—both the \(\mathrm{D}\)’s add from the same side.