19.6: Effect of Concentration
- Page ID
- 53909
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Phenolphthalein is a chemical that has one structure in a high acid environment, and another structure in a low acid environment. If the hydrogen ion concentration is high, the compound is colorless, but turns red if the hydrogen ion concentration is low. By adding hydrogen ions to the solution, or removing them through a chemical reaction, we can vary the color of the dye.
Effect of Concentration
A change in concentration of one of the substances in an equilibrium system typically involves either the addition or the removal of one of the reactants or products. Consider the Haber-Bosch process for the industrial production of ammonia from nitrogen and hydrogen gases:
\[\ce{N_2} \left( g \right) + 3 \ce{H_2} \left( g \right) \rightleftharpoons 2 \ce{NH_3} \left( g \right)\nonumber \]
If the concentration of one substance in a system is increased, the system will respond by favoring the reaction that removes that substance. When more \(\ce{N_2}\) is added, the forward reaction will be favored because the forward reaction uses up \(\ce{N_2}\) and converts it to \(\ce{NH_3}\). The forward reaction speeds up temporarily as a result of the addition of a reactant. The position of equilibrium shifts as more \(\ce{NH_3}\) is produced. The concentration of \(\ce{NH_3}\) increases, while the concentrations of \(\ce{N_2}\) and \(\ce{H_2}\) decrease. After some time passes, equilibrium is reestablished with new concentrations of all three substances. As can be seen in the figure below, if more \(\ce{N_2}\) is added, a new equilibrium is achieved by the system. The new concentration of \(\ce{NH_3}\) is higher because of the favoring of the forward reaction. The new concentration of the \(\ce{H_2}\) is lower. The concentration of \(\ce{N_2}\) is higher than in the original equilibrium, but went down slightly following the addition of the \(\ce{N_2}\) that disturbed the original equilibrium. By responding in this way, the value of the equilibrium constant for the reaction, \(K_\text{eq}\), does not change as a result of the stress to the system.
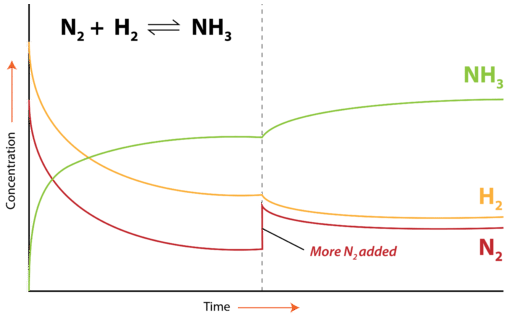
If more \(\ce{NH_3}\) were added, the reverse reaction would be favored. This "favoring" of a reaction means temporarily speeding up the reaction in that direction until equilibrium is reestablished. Recall that once equilibrium is reestablished, the rates of the forward and reverse reactions are again equal. The addition of \(\ce{NH_3}\) would result in increased formation of the reactants, \(\ce{N_2}\) and \(\ce{H_2}\).
An equilibrium can also be disrupted by the removal of one of the substances. If the concentration of a substance is decreased, the system will respond by favoring the reaction that replaces that substance. In the industrial Haber-Bosch process, \(\ce{NH_3}\) is removed from the equilibrium system as the reaction proceeds. As a result, the forward reaction is favored so that more \(\ce{NH_3}\) will be produced. The concentrations of \(\ce{N_2}\) and \(\ce{H_2}\) decrease. Continued removal of \(\ce{NH_3}\) will eventually force the reaction to go to completion until all of the reactants are used up. If either \(\ce{N_2}\) or \(\ce{H_2}\) were removed from the equilibrium system, the reverse reaction would be favored, and the concentration of \(\ce{NH_3}\) would decrease.
The effects of changes in concentration on an equilibrium system, according to Le Chatelier's principle, are summarized in the table below.
| Table \(\PageIndex{1}\) | |
|---|---|
| Stress | Response |
| Addition of reactant | Forward reaction favored. |
| Addition of product | Reverse reaction favored. |
| Removal of reactant | Reverse reaction favored. |
| Removal of product | Forward reaction favored. |

