5.4: Reactions of monosaccharides
- Page ID
- 423682
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Write and understand chemical reactions of monosaccharides, including conversion of cyclic hemiacetal to glycosides and N-glycosides, oxidation of open-chain aldehyde form to aldonic acids, and reduction to alditols.
- Define reducing sugars with the examples of D-glucose and D-fructose.
Glycosidic bond formation
Aldehydes and ketones react with alcohols to form hemiacetals. If the alcohol reagent is in excess, a second molecule of the alcohol reacts and converts hemiacetal to acetal. Acetals can be isolated. Acetals convert to alcohol and the aldehyde or ketone when their aqueous solution is acidified, as shown in the general reaction below.
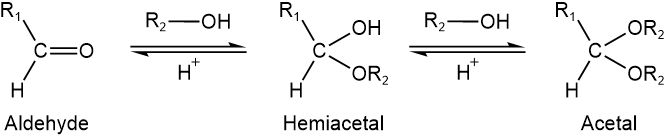
Cyclic (pyranose and furanose) forms of monosaccharides are hemiacetals that react with alcohols to form acetals.
Glycosides are the acetals of monosaccharides. The glucose formed by a reaction with alcohol is also called O-glycoside.
For example, \(\beta\)-D-glucopyranose reacts with methanol to form glycosides, shown in Figure \(\PageIndex{1}\).

An acetal of a monosaccharide is a glycoside. The bond between anomeric \(\ce{C}\) to the new ether \(\ce{-OR}\) group in the glycoside is called the glycosidic bond. Anomeric \(\ce{C}\) and glycosidic bonds are indicated by red arrows in Figure \(\PageIndex{1}\).
Glycoside is named by replacing the last e of the carbohydrate name with ide and adding the name of the alkyl part of the new ether \(\ce{-OR}\) group as a prefix separated by a hyphen. For example, \(\beta\)-D-glucopyranose reacts with methanol to form methyl-\(\beta\)-D-glucopyranoside and methyl-\(\alpha\)-D-glucopyranoside, as shown in Figure \(\PageIndex{1}\).
Since monosaccharides have many alcohol groups, two monosaccharides can react with each to form a glycoside, called a disaccharide, three can form a trisaccharide, and many can form a polysaccharide. The di-, tri-, and polysaccharides can be hydrolyzed to monosaccharides in an acidic aqueous solution.
A carbohydrate that can not be hydrolyzed to a simpler carbohydrate is a monosaccharide, e.g., D-glucose. A glycoside of two monosaccharides is a disaccharide, e.g., cellobiose. A glycoside of three to ten monosaccharides is an oligosaccharide, e.g., a fragment of cellulose. A glycoside of more than ten monosaccharides is a polysaccharide, e.g., cellulose. The figure below illustrates the structures of D-glucose -a monosaccharide; cellobiose -a disaccharide; and cellulose, -a polysaccharide.

Amines react with hemiacetals forms of monosaccharides the same way as alcohols and produce N-glycosides. For example, \(\beta\)-D-2-deoxyribose reacts with thymine and produced an N-glycoside called deoxythymidine, a part of a monomer of DNA molecule, as shown in the figure below.
 \(\beta\)-D-2-Deoxyribose (a monosaccharide)
\(\beta\)-D-2-Deoxyribose (a monosaccharide) Thymine (an amine)
Thymine (an amine) Doxythymidine (an N-glycoside)
Doxythymidine (an N-glycoside)The general formula of carbohydrates is \(\ce{C_{m}(H2O)_{n}}\), and usually for monosaccharides m is equal to n, i.e., \(\ce{C._{n}(H2O)_{n}}\) or \(\ce{C_{n}H_{2n}O_{n}}\). However, there are exceptions.
- Carbohydrates do not necessarily conform to this formula. For example, \(\beta\)-D-2-Deoxyribose \(\ce{C5H10O4}\) has less \(\ce{O's}\) than what is dictated by the general formula.
- Compounds following this general formula are not necessarily always carbohydrates. For example, acetic acid \(\ce{C2H4O2}\) follows the general formula, but it is not a carbohydrate.
Oxidation to aldonic acids
Aldehydes are oxidized by a variety of reagents to carboxylic acids. Similarly, the aldehyde group (\(\ce{-CHO}\)) of the open-chain form of aldoses is oxidized to a carboxylic acid (\(\ce{-COOH}\)) group by oxygen \(\ce{O2}\) in the presence of enzymes called oxidases. The acid is named by replacing the suffix -ose of the class name aldose with --onic acid. For example, D-glucose is oxidized to D-gluconic acid. Since, \(\ce{-COOH}\) exists in ionized (\(\ce{-COO^{-}}\)) form, the suffix -onic acid is replaced with -onate for the ionized form. For example, D-glucose is oxidized to D-gluconate, as shown in the figure below.
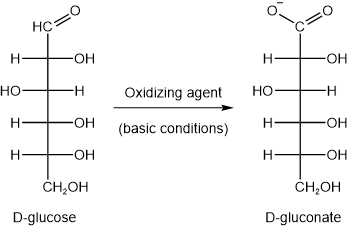
A carbohydrate that reacts with a mild oxidizing agent under alkaline conditions to form an aldonic acid (or aldonate anion in physiological conditions) is called a reducing sugar. For example, D-glucose is a reducing sugar, as shown in the figure above. The carbohydrate reduces the oxidizing agent.
Common reagents used to test the presence of a reducing sugar include Tollen's reagent in which \(\ce{Ag^{+}}\) is reduced to \(\ce{Ag}\) that forms a silver mirror on the glass, and Benedict's reagent in which \(\ce{Cu^{2+}}\) is reduced to \(\ce{Cu^{+}}\) that forms red color precipitate \(\ce{Cu2O}\), as shown in Figure \(\PageIndex{2}\).


Although open-chain form is in small concentration at equilibrium, it is continuously formed from the hemiacetal forms as the oxidation reaction removes it. The 2-ketoses are also reducing sugars. This is because 2-ketoses exist in equilibrium with the corresponding aldoses under the basic conditions. The aldose form is the actual reducing agent in this mixture. For example, D-fructose is a reducing sugar. D-fructose exists in equilibrium with D-glucose in basic conditions, where D-glucose is the reducing agent, as illustrated in the figure below.
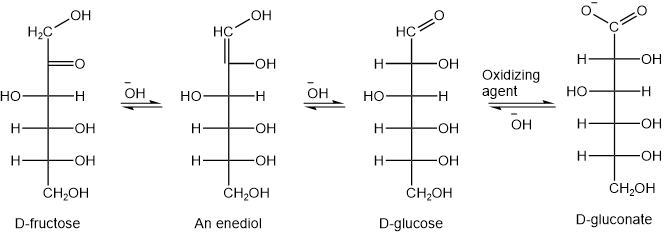
D-Glucose is also called blood sugar, as it is normally present in blood at about 70 mg/dL to 130 mg/dL. It may rise up to 140 mg/dL after eating food, but it returns to the normal range in healthy persons. Enzyme insulin controls blood sugar. If blood glucose control is not functioning properly, the blood glucose may stay higher than normal -a condition called hyperglycemia or diabetes; or it may stay below the normal level -a condition called hypoglycemia.
Blood glucose is usually tested based on enzyme-catalyzed reactions. Glucose oxidase enzyme oxidizes \(\beta\)-D-glucose using \(\ce{O2}\) to D-gluconate and hydrogen peroxide \(\ce{H2O2}\). \(\alpha\)-D-glucose does not react directly, but it converts to \(\beta\)-D-glucose as the latter is consumed. A second enzyme, peroxidase, causes \(\ce{H2O2}\) to react with 2-methylaniline and produces a colored product that is monitored to measure blood glucose, as illustrated in the figure below.


Reduction to alditols
Aldehydes are reduced to alcohols by a variety of reducing agents. Aldoses in the open-chain form are reduced by reducing agents like sodium borohydride (\(\ce{NaBH4}\)), \(\ce{H2}\) in the presence of \(\ce{Pt}\), \(\ce{Pd}\), or \(\ce{Ni}\). Oxidases enzymes are reducing agents in biochemical systems. Although open-chain aldehyde form is in small concentration in the equilibrium mixture, cyclic hemiacetal forms convert to open-chain form as the latter is consumed. The product alcohol is named by replacing -ose suffix of the aldose name with -itol. For example, D-glucose is reduced to D-glucitol, as shown below.
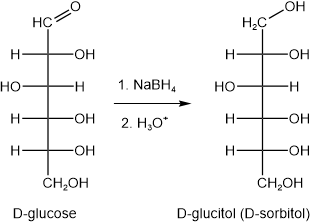

D-glucitol is found in berries, cherries, plums, pears, seaweed, and algae. It is commonly known as D-sorbitol and is a sugar substitute for diabetes. Other alditol examples include D-erythritol, D-mannitol, and D-xylitol. D-xylitol is used in cereals, sugarless candies, and gums.


