Reactions of Main Group Elements with Oxygen
- Page ID
- 606
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Oxygen is a highly reactive element that is very abundant on earth and in the human body. It is found in many compounds that are used to sustain basic life forms and modern civilization. Compounds containing oxygen are of great interest in the field of chemistry.
Background
Oxygen is ubiquitous; it comprises approximately 46% of the crust, 21% of the atmosphere, and 61% of the human body. Because of oxygen's high reactivity, it is most often found in compounds. Oxygen's high reactivity is due to its biradical electron configuration. As shown in a molecular orbital drawing of O2, the two unpaired electrons make the molecule highly susceptible to bond formation.
Oxygen has two allotropes (dioxygen, O2, and ozone, O3), both excellent oxidizing agents (Table P2). Oxygen is typically observed observed in the -2 oxidation state, in the form O2-, but it can also form other ions such as peroxide, O22-, and superoxide, O2-. With different possible oxidation states, many possible molecular compounds can be formed when an element reacts with oxygen. Many reactions involving oxygen occur in biological processes, including cellular respiration and photosynthesis.
Oxides are chemical compounds that contain at least one oxygen atom and at least one atom of another element. There are four principle oxidation states of oxygen: -2, -1, -1/2, and 0. The oxide ion, O2-, has a oxidation state of -2; the peroxide ion, O22-, has a oxidation state of -1; and the superoxide ion, O2-, has a oxidation state of -1/2. With metals, oxygen forms oxides that are largely ionic in character.
There are general trends in the reactions between main group elements and oxygen:
- Most nonmetals form the oxide with the highest possible oxidation state, with the halides excepted. Most metals form oxides with the oxygen in a -2 oxidation state.
- As a general rule, metal oxides are basic and nonmetal oxides are acidic. Basicity of an oxide increases with increasing ionic (metallic) character. Metal oxides, peroxides, and superoxides dissolve in water actually react with water to form basic solutions. Oxygen also forms covalent oxides with non-metals, that react with water to form acidic solutions.
- Oxygen does not react with fluorine or noble gases.
Exceptions to all of these trends are discussed below.
Reactions with Hydrogen
Oxygen reacts with hydrogen to produce two compounds: water (\(H_2O\)) and hydrogen peroxide (\(H_2O_2\)). Water is a versatile compound and participates in acid-base equilibrium and oxidation-reduction reactions. It can act as an acid, base, reducing agent, or oxidizing agent. Water's multifaceted abilities make it one of the most important compounds on earth. The reaction between hydrogen and oxygen to form water is given below:
\[2H_{2 (g)} + O_{2 (g)} \rightarrow 2H_2O_{(l)} \label{1} \]
Hydrogen peroxide's potent oxidizing abilities give it great industrial potential. The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide:
\[H_2 + O_2 \rightarrow H_2O_2 \label{2} \]
The product of this reaction is called a peroxide because oxygen is in the \(O_2^{2-}\) form (hydrogen has a +1 oxidation state). This concept is further explained regarding lithium below.
Reactions with Group 1 Elements
Oxygen reacts rapidly with Group 1 elements. All alkali metal oxides form basic solutions when dissolved in water. The principal combustion product is the most stable product with respect to the reactants. For example, with careful control of oxygen, the oxide M2O (where M represents any alkali metal) can be formed with any of the alkali metals. When heated, lithium, sodium, potassium, rubidium, and cesium ignite through combustion reactions with oxygen.
Lithium, the first metal in Group 1, reacts with oxygen to form Li2O and burns with a red flame. The oxygen in this compound is an oxide (O2-). The formation of Li2O, the principal combustion product, is illustrated by the equation below:
\[4 Li(s) + O_2(g) \rightarrow 2 Li_2O(s)\label{3} \]
However, if there is excess oxygen present, it is possible that a small amount of the compound Li2O2 can be formed. Because alkali metals always have a +1 oxidation state, oxygen is in the O22- form. When oxygen is in this state, the compound is called a peroxide. The formation of this peroxide, the less-likely non-principal combustion product, under excess oxygen is illustrated by the equation below:
\[2 Li(s) + O_2(g) \rightarrow Li_2O_2(s) \label{4} \]
Sodium burns in air with often little more than an orange glow. Using larger amounts of sodium or burning it in pure oxygen produces a strong orange flame. A white solid mixture of sodium oxide and sodium peroxide is formed. The equation for the formation of the simple oxide is analogous to that for lithium:
\[ 4Na(s) + O_2 (g) \rightarrow 2Na_2O (s) \label{5} \]
Likewise, the reaction for peroxide formation takes the same form for both metals:
\[ 2Na(s) + O_2 (g) \rightarrow Na_2O_2 (s) \label{6} \]

Small pieces of potassium heated in air tend to melt instantly into a mixture of potassium peroxide and potassium superoxide with no visible flame. Larger pieces of potassium burn with a lilac-colored flame. The equation for the formation of the peroxide is identical to that for sodium (click here for more information):
\[ 2K(s) + O_2 (g) \rightarrow K_2O_2 (s) \label{7} \]
The superoxide generating reaction is given below:
\[ K(s) + O_2 (g) \rightarrow KO_2 (s) \label{8} \]
Other alkali metals
The other alkali metals (Rb, Cs, Fr) form superoxide compounds (in which oxygen takes the form O2-) as the principal combustion products. The following equation shows the formation of superoxide, where M represents K, Rb, Cs, or Fr:
\[M(s) + O_2(g) \rightarrow MO_2(s) \label{9} \]
These compounds tend to be effective oxidizing agents due to the fact that O2- is one electron short of a complete octet and thus has a strong affinity for another electron. It is easily reduced, and therefore act as an effective oxidizing agent.
Reactions with Group 2 Elements
The elements of Group 2 are beryllium, magnesium, calcium, strontium, barium, and radioactive radium. Alkaline earth metals also react with oxygen, though not as rapidly as Group 1 metals; these reactions also require heating. Similarly to Group 1 oxides, most group 2 oxides and hydroxides are only slightly soluble in water and form basic, or alkaline solutions.
All Group 2 metals all react similarly, burning to form oxides (compounds containing the O2- ion) as shown:
\[2 M(s) + O_2(g) \rightarrow 2 MO(s) \label{10} \]
Once initiated, the reactions with oxygen are vigorous. The only peroxides (compounds containing the O22- ion) that can be formed from alkaline metals are strontium peroxide and barium peroxide. Both reactions require heat and excess oxygen. The general reaction is given below:
\[M(s) + O_2(g) \rightarrow MO_2(s)\label{11} \]
where M represents Sr or Ba.
Beryllium is unreactive with air and water. The chemical behavior of beryllium is best attributed to its small size and high ionization energy of its atoms.
All other group 2 metals
Except beryllium, the other alkaline earth metals form oxides in air at room temperature.
\[2 M(s) + O_2(g) \rightarrow 2 MO(s) \label{12} \]
where M represents Be, Mg, Ca, Sr, Ba, or Ra.
Peroxides, of the form MO2, are formed for all these elements except beryllium as shown:
\[M(s) + O_2(g) \rightarrow MO_2(s) \label{13} \]
Magnesium, calcium, strontium and barium oxides react with water to form hydroxides:
\[ MO(s) + H_2O(l) \rightarrow M(OH)_2(s) \label{14} \]
All the oxides and hydroxides of the group 2 metals, except of those of beryllium, are bases:
\[ M(OH)_2(s) \rightarrow M^{2+}(aq) + 2OH^-(aq) \label{15} \]
Reactions with Group 13 Elements
Group 13 consists of the following elements: boron, aluminum, gallium, indium, and thallium. Boron is the only element in this group that possesses no metallic properties. These elements vary in their reactions with oxygen. Recall that oxides of metals are basic and oxides or nonmetals are acidic; this is true for all elements in Group 13, except Al and Ga.
All other Group 13 elements also produce compounds of the form of M2O3, but adhere to the acid-base rules of metal and nonmetal oxides. Here is the equation of the reaction of oxygen and a Group 13 element:
\[4M(s) + 3O_2(g) \rightarrow 2M_2O_3(s) \label{16} \]
where M is any Group 13 element. At high temperatures, thallium also reacts with oxygen to produce Tl2O:
\[4Tl(s) + O_2(g) \rightarrow 2Tl_2O \label{17} \]
The most common oxide form of boron, B2O3 or boron trioxide, is obtained by heating boric acid:
\[2B(OH)_3 \xrightarrow{\Delta} B_2O_3 + 3H_2O \label{18} \]
Aluminum:
Aluminum occurs almost exclusively in the +3 oxidation state. It rapidly reacts with oxygen in air to give a water-insoluble coating of Al2O3. This oxide layer protects the metal beneath from further corrosion. The reaction is shown below:
\[4Al(s) + 3O_2(g) \rightarrow 2Al_2O_3 \label{19} \]
Aluminum trioxide, Al2O3, is amphoteric (acts both as an acid and a base):
\[Al_2O_3(s) + 6HCl(aq) \rightarrow 2AlCl_3(aq) + 3H_2O(l) \label{20} \]
\[Al_2O_3(s) + 2NaOH(aq) + 3H_2O(l) \rightarrow 2Na[Al(OH)_4](aq) \label{21} \]
Except for thallium in which the +1 oxidation state is more stable than the +3 state, aluminum, gallium, and indium favor +3 oxidation states
All of group 13 metal elements are known to form a trivalent oxide.
\[ 4M(s) + 3O_2(g) \rightarrow 2M_2O_3(s) \label{22} \]
with M represents Al, Ga, In, or Tl
Thallium is the only element in this group favors the formation of oxide over trioxide.
\[2M(s) + O_2(g) \rightarrow 2MO(s) \label{23} \]
Reactions with Group 14 Elements
Group 14 is made up of both metals (toward the bottom of the group), metalloids, and nonmetals (at the top of the group). The oxides of the top of Group 4 elements are slightly acidic, and the acidity of the oxides decreases down the group.
- Non-metal: The non-metal carbon of Group 14 (and its compounds) burn to form \(CO_{2\;(g)}\) and, in smaller amounts, \(CO_{(g)}\); both are acidic under different conditions. Carbon monoxide is only slightly soluble in water and does not react with it. Click here for more Information.
- Metalloid: The metalloid silicon reacts with oxygen to form only one stable compound, \(SiO_{2}\), which dissolves slightly in water and is weakly acidic(Figure 2).
- The three metals in this group have many different oxide compounds due to their extended octets. All of these oxides are amphoteric (exhibit both basic and acidic properties). For example:
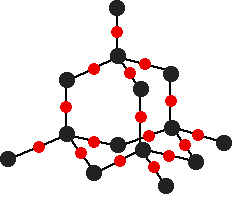
Reactions with Group 15 Elements
The nitrogen family, Group 15, is capable of reacting with oxygen in many different ways. Nitrogen and phosphorus are nonmetallic, arsenic and antimony are metalloids, and bismuth is metallic.
Nitrogen reacts with oxygen to form many oxides ranging in oxidation states from +1 to +5: All these oxides are gases at room temperature except for N2O5, which is solid. The nitrogen oxides are given below:
NO, N2O, N2O3, NO2, N2O5
All of these reactions are endothermic, requiring energy for oxygen to react directly with N2(g). The oxides of nitrogen are acidic (because they are nonmetal oxides). N2O3 and N2O5 react with water to give acidic solutions of oxoacids. These reactions are shown below:
Nitrous acid: \[N_2O_3(s) + H_2O(l) \rightarrow 2HNO_2(aq) \label{24} \]
Nitric acid: \[N_2O_5(s) + H_2O(l) \rightarrow 2HNO_3(aq) \label{25} \]
There are two forms of allotropes of phosphorus, white phosphorus and red phosphorus. Red phosphorus is less reactive than white phosphorus. Phosphorus reacts with oxygen, usually forming two oxides depending on the amount available oxygen: P4O6 when reacted with a limited supply of oxygen, and P4O10 when reacted with excess oxygen; the latter is shown below.
\[P_4O_{10} + 6 H_2O \rightarrow 4 H_3PO \label{26} \]
On rare occasions, P4O7, P4O8, and P4O9 are also formed, but in small amounts.
Both P4O4 and P4O10 react with water to generate oxoacids. Reactions are shown below.
Phosphorous acid: \[P_4O_6(l) + 6H_2O(l) \rightarrow 4H_3PO_3(aq) \label{27} \]
Phosphoric acid: \[P_4O_{10}(s) + 6H_2O(l) \rightarrow 4H_3PO_4(aq) \label{28} \]
Other Group 15 Elements
Arsenic, antimony and bismuth react with oxygen when burned. The common oxidation states for arsenic, antimony, and bismuth are +3 and +5. There are two main types of oxides for each element:
- Arsenic: As2O3, As2O5
- Antimony: Sb2O3, Sb2O5
- Bismuth: Bi2O3, Bi2O5
There are other oxides, such as Sb4O10, that are not formed directly through reaction with oxygen. Arsenic(III) oxide and antimony(III) oxide are amphoteric, whereas bismuth(III) oxide acts only as a base (this is because it is the most metallic element in the group).
Reactions with Group 16 Elements
The elements in Group 16 include oxygen, sulfur, selenium, tellurium, and polonium. Oxygen reacts with the elements in its own group to form various oxides, mostly in the form of AO2 and AO3.
Although oxygen is located in Group 16, it is unique in its extreme electronegativity; this allows it to readily gain electrons and create hydrogen bonds. Because it is the smallest element in its group, it is capable of forming double bonds. It has no d-orbitals, and cannot expand its valence shell.
Oxygen is capable of reacting with itself, forming allotropes. One of oxygen's allotropes, ozone (O3), is formed when oxygen gas, O2, is subjected to ultraviolet light.
Sulfur dioxide, SO2, and sulfur trioxide, SO3, are the only common sulfur oxides.
\[S(s) + O_2(g) \xrightarrow{\Delta} SO_2(g) \label{29} \]
Sulfur's reaction with oxygen produces the oxides mentioned above as well as oxoacids. All are powerful oxidizing agents. SO2 is mainly used to make SO3, which reacts with water to produce sulfuric acid (recall that nonmetals form acidic oxides). These sequential reactions are shown below:
\[ 2SO_2(g) + O_2(g) \rightleftharpoons 2SO_3(g) \label{30} \]
\[2SO_3(g) + H_2O(l) \rightarrow H_2SO_4(aq) \label{31} \]
Selenium and tellurium
Selenium and tellurium adopt compounds of the forms AO2, AO3, and AO.
Reactions with Group 17 Elements
The elements in Group 17 include fluorine, chlorine, bromine, and iodine. These elements are called halogens, from Greek roots translating to "salt formers." The halogens react with oxygen, but many of the resulting compounds are unstable, lasting for only moments at a time. They range in structure from X2O to X2O7, where X represents a halogen. Their extended octets allow them to bond with many oxygen atoms at a time.
The most electronegative element adopts the -1 oxidation state. Fluorine and oxygen form OF2, which is known as oxygen fluoride.
\[2 F_2 + 2NaOH \rightarrow OF_2 + 2 NaF + H_2O \label{32} \]
Other Halogens
The other halogens form oxoacids instead of oxides. For example:
| Oxidation state of halogen | Chlorine | Bromine | Iodine |
|---|---|---|---|
| +1 | HOCl | HOBr | HOI |
| +3 | HClO2 | –— | –— |
| +5 | HClO3 | HBrO3 | HIO HIO3 |
| +7 | HClO4 | HBrO4 | HIO4; H5IO6 |
Reactions with Group 18 Elements
The Group 18 noble gases include helium, neon, krypton, xenon, and radon. Noble gases are chemically inert with the exception of xenon, which reacts with oxygen to form XeO3 and XeO4 at low temperatures and high pressures. The ionization energy of xenon is low enough for the electronegative oxygen atom to capture electrons. XeO3 is highly unstable, and is known to spontaneously detonate in a clean, dry environment.
References
- Reactions of Selenium and Oxygen. Matrix Infrared Spectra and Density Functional Calculations of Novel SexOy Molecule
- G. Dana Brabson and, Lester Andrews, Colin J. Marsden. The Journal of Physical Chemistry 1996 100 (41), 16487-16494
- Petrucci, Ralph H. General Chemistry: Principles and Modern Applications - 9th ed. Upper Saddle River: Prentice Hall, 2007. Print.
- Zumdahl, Steven S. Chemical Principles - 5th ed. Houghton Mifflin Company, 2005. Print.
- Madsen, Dorte. Class Lecture Notes. Main Group Elements. University of California, Davis, California. Spring 2011.
Problems
- If there are 4.00 g of potassium, how much K2O is created when during combustion (in excess oxygen)?
- Which noble gas(es), if any, react with oxygen? Why?
- Complete and balance the following reaction: Al(s) + O2(g) →
- What is the best environment for nitrogen and oxygen to react in?
- What are the principal combustion products of each Group 1 element with oxygen?
Solutions
- 0.00 g K2O. Potassium reacts with oxygen to form K2O2 and KO2 only.
- Xenon, because the first ionization energy is low enough, allowing oxygen to bond to the xenon atom.
- 4Al(s) + 3O2(g) → Al2O3(s)
- High temperatures - it takes energy to cause those reactions.
- Li generally forms Li2O. Na usually forms Na2O2. The rest of Group 1 elements tend to form comppunds in the form of MO2 where M is K, Rb, Cs, or Fr.
Contributors and Attributions
- Kathleen M. (UCD Spring 2010), Antoinette Mursa (UC Davis Spring 2011)

