Atomic and Physical Properties of Halogens
- Page ID
- 3696
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)This page discusses the trends in the atomic and physical properties of the Group 7 elements (the halogens): fluorine, chlorine, bromine and iodine. Sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility, including a discussion of the bond enthalpies of halogen-halogen and hydrogen-halogen bonds.
Trends in Atomic Radius
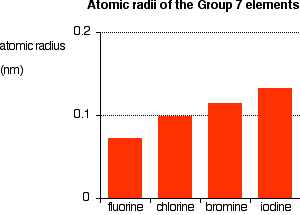
The figure above shows the increase in atomic radius down the group.
Explaining the increase in atomic radius
The radius of an atom is determined by:
- the number of layers of electrons around the nucleus
- the pull the outer electrons feel from the nucleus.
Compare the numbers of electrons in each layer of fluorine and chlorine:
| F | 2,7 | |
| Cl | 2,8,7 |
In each case, the outer electrons feel a net +7 charge from the nucleus. The positive charge on the nucleus is partially neutralized by the negative inner electrons.
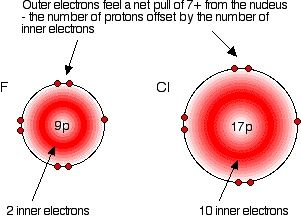
This is true for all the atoms in Group 7: the outer electrons experience a net charge of +7..
The only factor affecting the size of the atom is therefore the number of layers of inner electrons surrounding the atom. More layers take up more space due to electron repulsion, so atoms increase in size down the group.
Trends in Electronegativity
Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. It is usually measured on the Pauling scale, on which the most electronegative element (fluorine) is assigned an electronegativity of 4.0. The figure below shows electronegativities for each halogen:
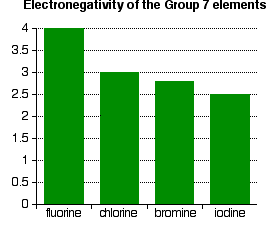
Notice that electronegativity decreases down the group. The atoms become less effective at attracting bonding pairs of electrons. This effect is illustrated below using simple dots-and-crosses diagrams for hydrogen fluoride and hydrogen chloride:
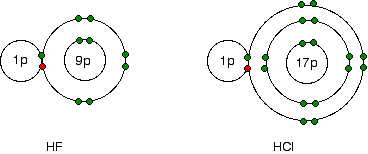
The bonding pair of electrons between the hydrogen and the halogen experiences the same net pull of +7 from both the fluorine and the chlorine. However, in the chlorine case, the nucleus is farther away from the bonding electrons, which are therefore not as strongly attracted as in the fluorine case.
The stronger attraction from the closer fluorine nucleus makes fluorine more electronegative than chlorine.
Summarizing the trend down the Group
As the halogen atoms increase in size, any bonding pair gets farther away from the halogen nucleus, and so is less strongly attracted toward it. Hence, down the group, the elements become less electronegative.
Trends in First Electron Affinity
The first electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of gaseous 1- ions. In other words, it is the energy released in the following process:
\[ X(g) + e^- \rightarrow X^- (g) \nonumber \]
First electron affinities have negative values by convention. For example, the first electron affinity of chlorine is -349 kJ mol-1. The negative sign indicates a release of energy.
The first electron affinities of the Group 7 elements
The electron affinity is a measure of the attraction between the incoming electron and the nucleus. There is a positive correlation between attraction and electron affinity. The trend down the group is illustrated below:
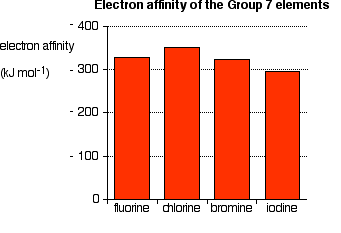
Notice that the trend down the group is inconsistent. The electron affinities generally decrease (meaning less heat is emitted), but the fluorine value deviates from this trend.

In the larger atom, the attraction from the more positive nucleus is offset by the additional screening electrons, so each incoming electron feels the effect of a net +7 charge from the center.
As the atom increases in size, the incoming electron is farther from the nucleus and so feels less attraction. The electron affinity therefore decreases down the group. However fluorine is a very small atom, with the incoming electron relatively close to the nucleus, and yet the electron affinity is smaller than expected.
Another effect must be considered in the case of fluorine. As the new electron comes approaches the atom, it enters a region of space already very negatively charged because of the existing electrons. The resulting repulsion from these electrons offsets some of the attraction from the nucleus.
Because the fluorine atom is very small, its existing electron density is very high. Therefore, the extra repulsion is particularly great and diminishes the attraction from the nucleus enough to lower the electron affinity below that of chlorine.
Trends in Melting Point and Boiling Point
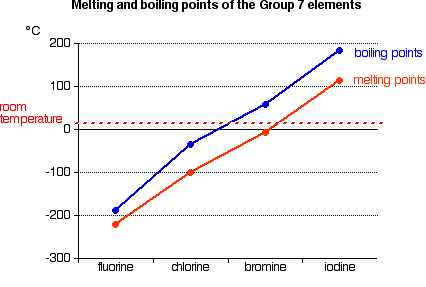
Melting and boiling points increase down the group. As indicated by the graph above, fluorine and chlorine are gases at room temperature, bromine is a liquid and iodine a solid.
Explaining the trends in melting point and boiling point
All the halogens exist as diatomic molecules—F2, Cl2, and so on. van der Waals dispersion forces are the primary intermolecular attractions between one molecule and its neighbors. Larger molecules farther down the group have more electrons which can move around and form the temporary dipoles that create these forces.
The stronger intermolecular attractions down the group require more heat energy for melting or vaporizing, increasing their melting or boiling points.
Solubilities
Solubility in water
Fluorine reacts violently with water to produce aqueous or gaseous hydrogen fluoride and a mixture of oxygen and ozone; its solubility is meaningless. Chlorine, bromine, and iodine all dissolve in water to some extent, but there is again no discernible pattern. The following table shows the solubility of the three elements in water at 25°C:
| solubility (mol dm-3) |
|
|---|---|
| chlorine | 0.091 |
| bromine | 0.21 |
| iodine | 0.0013 |
Chlorine dissolved in water produces a pale green solution. Bromine solution adopts a range of colors from yellow to dark orange-red depending on the concentration. Iodine solution in water is very pale brown. Chlorine reacts with water to some extent, producing a mixture of hydrochloric acid and chloric(I) acid (also known as hypochlorous acid). The reaction is reversible, and at any time only about a third of the chlorine molecules have reacted.
\[ Cl_2 + H_2O \rightleftharpoons HCl + HClO \nonumber \]
Chloric(I) acid is sometimes symbolized as HOCl, indicating the actual bonding pattern. Bromine and iodine form similar compounds, but to a lesser extent. In both cases, about 99.5% of the halogen remains unreacted.
The solubility of iodine in potassium iodide solution
Although iodine is only slightly soluble in water, it dissolves freely in potassium iodide solution, forming a dark red-brown solution. A reversible reaction between iodine molecules and iodide ions gives I3- ions. These are responsible for the color. In the laboratory, iodine is often produced through oxidation of iodide ions. As long as there are any excess iodide ions present, the iodine reacts to form I3-. Once the iodide ions have all reacted, the iodine is precipitated as a dark gray solid.
Solubility in hexane
The halogens are much more soluble in organic solvents such as hexane than they are in water. Both hexane and the halogens are non-polar molecules, so the only intermolecular forces between them are van der Waals dispersion forces. Because of this, the attractions broken (between hexane molecules and between halogen molecules) are similar to the new attractions made when the two substances mix. Organic solutions of iodine are pink-purple in color.
Bond enthalpies (bond energies or bond strengths)
Bond enthalpy is the heat required to break one mole of covalent bonds to produce individual atoms, starting from the original substance in the gas state, and ending with gaseous atoms. For chlorine, Cl2(g), it is the heat energy required for the following reaction, per mole:
\[ Cl-Cl (g) \rightarrow 2Cl(g) \nonumber \]
Although bromine is a liquid, the bond enthalpy is defined in terms of gaseous bromine molecules and atoms, as shown below:
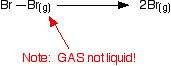
Bond enthalpy in the halogens, X2(g)
Covalent bonding is effective because the bonding pair is attracted to both the nuclei at either side of it. It is that attraction which holds the molecule together. The extent of the attraction depends in part on the distances between the bonding pair and the two nuclei. The figure below illustrates such a covalent bond:
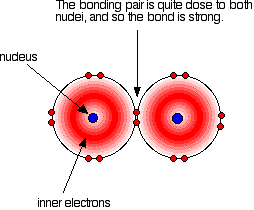
In all halogens, the bonding pair experiences a net +7 charge from either end of the bond, because the charge on the nucleus is offset by the inner electrons. As the atoms get larger down the group, the bonding pair is further from the nuclei and the strength of the bond should, in theory, decrease, as indicated in the figure below. The question is whether experimental data matches this prediction.
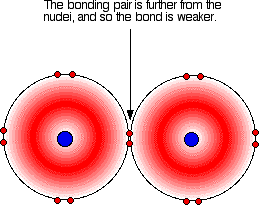
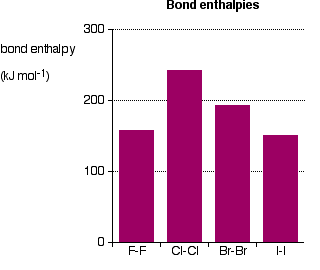
As is clear from the figure above, the bond enthalpies of the Cl-Cl, Br-Br and I-I bonds decreases as predicted, but the F-F bond enthalpy deviates.
Because fluorine atoms are so small, a strong bond is expected—in fact, it is remarkably weak. There must be another factor for consideration.
In addition to the bonding pair of electrons between the two atoms, each atom has 3 lone pairs of electrons in the outer shell. If the bond is very short,as in F-F, the lone pairs on the two atoms are close enough to cause significant repulsion, illustrated below:

In the case of fluorine, this repulsion is great enough to counteract much of the attraction between the bonding pair and the two nuclei. This weakens the bond.
Bond enthalpies in the hydrogen halides, HX(g)
If the halogen atom is attached to a hydrogen atom, this does not occur; there are no lone pairs on a hydrogen atom. Bond enthalpies for halogen-hydrogen bonds are given below:
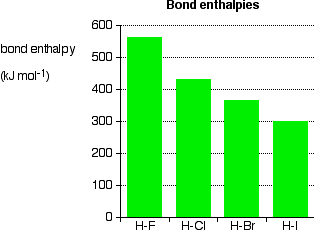
As larger halogens are involved, the bonding pair is more distant from the nucleus. The attraction is lessened, and the bond should be weaker; this is supported by the data, without exception. This fact has significant implications for the thermal stability of the hydrogen halides— they are easily broken into hydrogen and the halogen on heating.
Hydrogen fluoride and hydrogen chloride are thermally very stable under typical laboratory conditions. Hydrogen bromide breaks down to some extent into hydrogen and bromine on heating, and hydrogen iodide is even less stable when heated. Weaker bonds are more easily broken.
Contributors and Attributions
Jim Clark (Chemguide.co.uk)


