10.3: Acid-base reactions à la Brønsted
- Page ID
- 3489
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Make sure you thoroughly understand the following essential ideas which have been presented above. It is especially important that you know the precise meanings of all the highlighted terms in the context of this topic.
- Explain the difference between the Arrhenius and Bronsted-Lowry concepts of acids and bases, and give examples of an acid and a base in which the Arrhenius concept is inapplicable.
- Explain why a hydrogen ion cannot exist in water.
- Given the formula of an acid or base, write the formula of its conjugate.
- State the fundamental difference between a strong acid and a weak acid.
- Describe the leveling effect, and explain its origin.
- State the factors that determine whether a solution of a salt will be acidic or alkaline.
- Write the equation for the autoprotolysis of a given ampholyte.
In this lesson we develop this concept and illustrate its applications to "strong" and "weak" acids and bases, emphasizing the common theme that acid-base chemistry is always a competition between two bases for the proton. In the final section, we show how the concept of "proton energy" can help us understand and predict the direction and extent of common types of acid-base reactions without the need for calculations.
Proton donors and acceptors
The older Arrhenius theory of acids and bases viewed them as substances which produce hydrogen ions or hydroxide ions on dissociation. As useful a concept as this has been, it was unable to explain why NH3, which contains no OH– ions, is a base and not an acid, why a solution of FeCl3 is acidic, or why a solution of Na2S is alkaline. A more general theory of acids and bases was developed by Franklin in 1905, who suggested that the solvent plays a central role. According to this view, an acid is a solute that gives rise to a cation (positive ion) characteristic of the solvent, and a base is a solute that yields a anion (negative ion) which is also characteristic of the solvent. The most important of these solvents is of course H2O, but Franklin's insight extended the realm of acid-base chemistry into non-aqueous systems as we shall see in a later lesson.
Brønsted acids and bases
In 1923, the Danish chemist J.N. Brønsted, building on Franklin's theory, proposed that an acid is a proton donor; a base is a proton acceptor. In the same year the English chemist T.M. Lowry published a paper setting forth some similar ideas without producing a definition; in a later paper Lowry himself points out that Brønsted deserves the major credit, but the concept is still widely known as the Brønsted-Lowry theory.
Brønsted-Lowry Acids and Bases
An acid is a proton donor and a base is a proton acceptor.These definitions carry a very important implication: a substance cannot act as an acid without the presence of a base to accept the proton, and vice versa. As a very simple example, consider the equation that Arrhenius wrote to describe the behavior of hydrochloric acid:
\[HCl \rightarrow H^+ + A^–\]
This is fine as far as it goes, and chemists still write such an equation as a shortcut. But in order to represent this more realistically as a proton donor-acceptor reaction, we now depict the behavior of HCl in water by

in which the acid HCl donates its proton to the acceptor (base) H2O.
"Nothing new here", you might say, noting that we are simply replacing a shorter equation by a longer one. But consider how we might explain the alkaline solution that is created when ammonia gas NH3 dissolves in water. An alkaline solution contains an excess of hydroxide ions, so ammonia is clearly a base, but because there are no OH– ions in NH3, it is clearly not an Arrhenius base. It is, however, a Brønsted base:
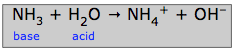
In this case, the water molecule acts as the acid, donating a proton to the base NH3 to create the ammonium ion NH4+.
The foregoing examples illustrate several important aspects of the Brønsted-Lowry concept of acids and bases:
- A substance cannot act as an acid unless a proton acceptor (base) is present to receive the proton;
- A substance cannot act as a base unless a proton donor (acid) is present to supply the proton;
- Water plays a dual role in many acid-base reactions; H2O can act as a proton acceptor (base) for an acid, or it can serve as a proton donor (acid) for a base (as we saw for ammonia.
- The hydronium ion H3O+ plays a central role in the acid-base chemistry of aqueous solutions.
Brønsted
Brønsted (1879-1947) was a Danish physical chemist. Although he is now known mainly for his proton donor-acceptor theory of acids and bases (see his original article), he published numerous earlier papers on chemical affinity, and later on the catalytic effects of acids and bases on chemical reactions. In World War II he opposed the Nazis, and this led to his election to the Danish parliament in 1947, but he was unable to take his seat because of illness and he died later in that year.
Lowry
Lowry (1874-1936) was the first holder of the chair in physical chemistry at Cambridge University. His extensive studies of the effects of acids and bases on the optical behavior of camphor derivatives (specifically, how they rotate the plane of polarized light) led him to formulate a theory of acids and bases similar to and simultaneously with that of Brønsted.
The Hydronium ion
There is another serious problem with the Arrhenius view of an acid as a substance that dissociates in water to produce a hydrogen ion. The hydrogen ion is no more than a proton, a bare nucleus. Although it carries only a single unit of positive charge, this charge is concentrated into a volume of space that is only about a hundred-millionth as large as the volume occupied by the smallest atom. (Think of a pebble sitting in the middle of a sports stadium!) The resulting extraordinarily high charge density of the proton strongly attracts it to any part of a nearby atom or molecule in which there is an excess of negative charge. In the case of water, this will be the lone pair (unshared) electrons of the oxygen atom; the tiny proton will be buried within the lone pair and will form a shared-electron (coordinate) bond with it, creating a hydronium ion, H3O+. In a sense, H2O is acting as a base here, and the product H3O+ is the conjugate acid of water:
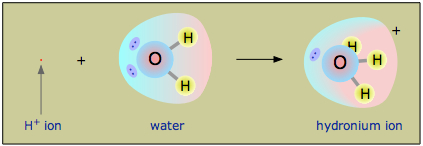
Owing to the overwhelming excess of \(H_2O\) molecules in aqueous solutions, a bare hydrogen ion has no chance of surviving in water.
Although other kinds of dissolved ions have water molecules bound to them more or less tightly, the interaction between H+ and H2O is so strong that writing “H+(aq)” hardly does it justice, although it is formally correct. The formula H3O+ more adequately conveys the sense that it is both a molecule in its own right, and is also the conjugate acid of water.
The equation "HA → H+ + A–" is so much easier to write that chemists still use it to represent acid-base reactions in contexts in which the proton donor-acceptor mechanism does not need to be emphasized. Thus it is permissible to talk about “hydrogen ions” and use the formula H+ in writing chemical equations as long as you remember that they are not to be taken literally in the context of aqueous solutions.
Interestingly, experiments indicate that the proton does not stick to a single H2O molecule, but changes partners many times per second. This molecular promiscuity, a consequence of the uniquely small size and mass the proton, allows it to move through the solution by rapidly hopping from one H2O molecule to the next, creating a new H3O+ ion as it goes. The overall effect is the same as if the H3O+ ion itself were moving. Similarly, a hydroxide ion, which can be considered to be a “proton hole” in the water, serves as a landing point for a proton from another H2O molecule, so that the OH– ion hops about in the same way.
Because hydronium- and hydroxide ions can “move without actually moving” and thus without having to plow their way through the solution by shoving aside water molecules as do other ions, solutions which are acidic or alkaline have extraordinarily high electrical conductivities.
Acid-base reactions à la Brønsted
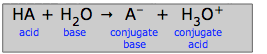
According to the Brønsted concept, the process that was previously written as a simple dissociation of a generic acid HA ("HA → H+ + A–)" is now an acid-base reaction in its own right:
\[HA + H_2O \rightarrow A^- + H_3O^+\]
The idea, again, is that the proton, once it leaves the acid, must end up somewhere; it cannot simply float around as a free hydrogen ion.
Conjugate pairs
A reaction of an acid with a base is thus a proton exchange reaction; if the acid is denoted by AH and the base by B, then we can write a generalized acid-base reaction as
\[AH + B \rightarrow A^- + BH^+\]
Notice that the reverse of this reaction,
\[BH^+ + A^- \rightarrow B + AH^+\]
is also an acid-base reaction. Because all simple reactions can take place in both directions to some extent, it follows that transfer of a proton from an acid to a base must necessarily create a new pair of species that can, at least in principle, constitute an acid-base pair of their own.
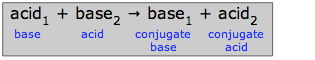
In this schematic reaction, base1 is said to be conjugate to acid1, and acid2 is conjugate to base2. The term conjugate means “connected with”, the implication being that any species and its conjugate species are related by the gain or loss of one proton. The table below shows the conjugate pairs of a number of typical acid-base systems.
|
acid
|
base
|
||
|---|---|---|---|
| hydrochloric acid | HCl | Cl– | chloride ion |
| acetic acid | CH3CH2COOH | CH3CH2COO– | acetate ion |
| nitric acid | HNO3 | NO3– | nitrate ion |
| dihydrogen phosphate ion | H2PO4– | HPO4– | monohydrogen phosphate ion |
| hydrogen sulfate ion | HSO4– | SO42– | sulfate ion |
| hydrogen carbonate ("bicarbonate") ion | HCO3– | CO32– | carbonate ion |
| ammonium ion | NH4+ | NH3 | ammonia |
| iron(III) ("ferric") ion | Fe(H2O)63+ | Fe(H2O)5OH2+ | |
| water | H2O | OH– | hydroxide ion |
| hydronium ion | H3O+ | H2O | water |
Strong acids and weak acids
We can look upon the generalized acid-base reaction
as a competition of two bases for a proton:

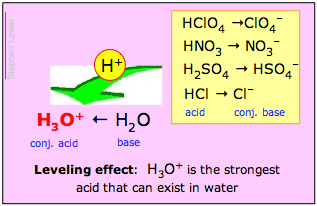
If the base H2O overwhelmingly wins this tug-of-war, then the acid HA is said to be a strong acid. This is what happens with hydrochloric acid and the other common strong "mineral acids" H2SO4, HNO3, and HClO4:
| hydrochloric acid | HCl + H2O → Cl– + H3O+ |
| sulfuric acid | H2SO4 + H2O → HSO4– + H3O+ |
| nitric acid | HNO3 + H2O → NO3– + H3O+ |
| perchloric acid | HClO4 + H2O → ClO4– + H3O+ |
Solutions of these acids in water are really solutions of the ionic species shown in heavy type on the right. This being the case, it follows that what we call a 1 M solution of "hydrochloric acid" in water, for example, does not really contain a significant concentration of HCl at all; the only real a acid present in such a solution is H3O+!
These considerations give rise to two important rules:
- H3O+ is the strongest acid that can exist in water;
- All strong acids appear to be equally strong in water.
The Leveling Effect
The second of these statements is called the leveling effect. It means that although the inherent proton-donor strengths of the strong acids differ, they are all completely dissociated in water. Chemists say that their strengths are "leveled" by the solvent water.
A comparable effect would be seen if one attempted to judge the strengths of several adults by conducting a series of tug-of-war contests with a young child. One would expect the adults to win overwhelmingly on each trial; their strengths would have been "leveled" by that of the child.
Weak acids
Most acids, however, are able to hold on to their protons more tightly, so only a small fraction of the acid is dissociated. Thus hydrocyanic acid, HCN, is a weak acid in water because the proton is able to share the lone pair electrons of the cyanide ion CN– more effectively than it can with those of H2O, so the reaction
\[HCN + H_2O \rightarrow H_3O^+ + CN^–\]
proceeds to only a very small extent. Since a strong acid binds its proton only weakly, while a weak acid binds it tightly, we can say that
Strong acids are "weak" and weak acids are "strong." If you are able to explain this apparent paradox, you understand one of the most important ideas in acid-base chemistry!
|
reaction
|
acid
|
base
|
conjugate acid
|
conjugate base
|
|---|---|---|---|---|
| autoionization of water H2O | H2O | H2O | H3O+ | OH– |
| ionization of hydrocyanic acid HCN | HCN | H2O | H3O+ | CN– |
| ionization of ammonia NH3 in water | NH3 | H2O | NH4+ | OH– |
| hydrolysis of ammonium chloride NH4Cl | NH4+ | H2O | H3O+ | NH3 |
| hydrolysis of sodium acetate CH3COO- Na+ | H2O | CH3COO– | CH3COOH | OH– |
| neutralization of HCl by NaOH | HCl | OH– | H2O | Cl– |
| neutralization of NH3 by acetic acid | CH3COOH | NH3 | NH4+ | CH3COO– |
| dissolution of BiOCl (bismuth oxychloride) by HCl | 2 H3O+ | BiOCl | Bi(H2O)3+ | H2O, Cl– |
| decomposition of Ag(NH3)2+ by HNO3 | 2 H3O+ | Ag(NH3)2+ | NH4+ | H2O |
| displacement of HCN by CH3COOH | CH3COOH | CN– | HCN | CH3COO– |
Strong acids have weak conjugate bases
This is just a re-statement of what is implicit in what has been said above about the distinction between strong acids and weak acids. The fact that HCl is a strong acid implies that its conjugate base Cl– is too weak a base to hold onto the proton in competition with either H2O or H3O+. Similarly, the CN– ion binds strongly to a proton, making HCN a weak acid.
Salts of weak acids give alkaline solutions
The fact that HCN is a weak acid implies that the cyanide ion CN– reacts readily with protons, and is thus is a relatively good base. As evidence of this, a salt such as KCN, when dissolved in water, yields a slightly alkaline solution:
CN– + H2O → HCN + OH–
This reaction is still sometimes referred to by its old name hydrolysis ("water splitting"), which is literally correct but tends to obscure its identity as just another acid-base reaction. Reactions of this type take place only to a small extent; a 0.1M solution of KCN is still, for all practical purposes, 0.1M in cyanide ion.
In general, the weaker the acid, the more alkaline will be a solution of its salt. However, it would be going to far to say that "ordinary weak acids have strong conjugate bases." The only really strong base is hydroxide ion, OH–, so the above statement would be true only for the very weak acid H2O.
Strong bases and weak bases
The only really strong bases you are likely to encounter in day-to-day chemistry are alkali-metal hydroxides such as NaOH and KOH, which are essentially solutions of the hydroxide ion. Most other compounds containing hydroxide ions such as Fe(OH)3 and Ca(OH)2 are not sufficiently soluble in water to give highly alkaline solutions, so they are not usually thought of as strong bases.
There are actually a number of bases that are stronger than the hydroxide ion — best known are the oxide ion O2– and the amide ion NH2–, but these are so strong that they can rob water of a proton:
O2– + H2O → 2 OH–
NH2– + H2O → NH3 + OH–
This gives rise to the same kind of leveling effect we described for acids, with hydroxide ion as the strongest base in water.
Hydroxide ion is the strongest base that can exist in aqueous solution.
The most common example of this is ammonium chloride, NH4Cl, whose aqueous solutions are distinctly acidic:
NH4+ + H2O → NH3 + H3O+
Because this (and similar) reactions take place only to a small extent, a solution of ammonium chloride will only be slightly acidic.
Autoprotolysis
From some of the examples given above, we see that water can act as an acid
CN– + H2O → HCN + OH–
and as a base
NH4+ + H2O → NH3 + H3O+
If this is so, then there is no reason why "water-the-acid" cannot donate a proton to "water-the-base":

This reaction is known as the autoprotolysis of water.
Chemists still often refer to this reaction as the "dissociation" of water and use the Arrhenius-style equation H2O → H+ + OH–as a kind of shorthand. As discussed in the previous lesson, this process occurs to only a tiny extent. It does mean, however, that hydronium and hydroxide ions are present in any aqueous solution.
Ammonia and Sulfuric acid
Other liquids also exhibit autoprotolysis with the most well-known example is liquid ammonia:
2 NH3 → NH4+ + NH2–
Even pure liquid sulfuric acid can play the game:
2 H2SO4→ H3SO4+ + HSO4–
Each of these solvents can be the basis of its own acid-base "system", parallel to the familiar "water system".
Ampholytes
Water, which can act as either an acid or a base, is said to be amphiprotic: it can "swing both ways". A substance such as water that is amphiprotic is called an ampholyte.
As indicated here, the hydroxide ion can also be an ampholyte, but not in aqueous solution in which the oxide ion cannot exist.
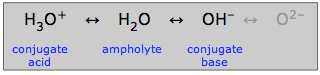
It is of course the amphiprotic nature of water that allows it to play its special role in ordinary aquatic acid-base chemistry. But many other amphiprotic substances can also exist in aqueous solutions. Any such substance will always have a conjugate acid and a conjugate base, so if you can recognize these two conjugates of a substance, you will know it is amphiprotic.
The carbonate system
For example, the triplet set {carbonic acid, bicarbonate ion, carbonate ion} constitutes an amphiprotric series in which the bicarbonate ion is the ampholyte, differing from either of its neighbors by the addition or removal of one proton:
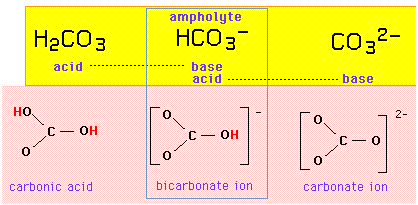
If the bicarbonate ion is both an acid and a base, it should be able to exchange a proton with itself in an autoprotolysis reaction:
\[HCO_3^– + HCO_3^– \rightarrow H_2CO_3 + CO_3^{2-}\]
Carbonic Acid
Your very life depends on the above reaction! CO2, a metabolic by-product of every cell in your body, reacts with water to form carbonic acid H2CO3 which, if it were allowed to accumulate, would make your blood fatally acidic. However, the blood also contains carbonate ion, which reacts according to the reverse of the above equation to produce bicarbonate which can be safely carried by the blood to the lungs. At this location the autoprotolysis reaction runs in the forward direction, producing H2CO3 which loses water to form CO2 which gets expelled in the breath. The carbonate ion is recycled back into the blood to eventually pick up another CO2 molecule.
If you can write an autoprotolysis reaction for a substance, then that substance is amphiprotic.


