9.5: Energetics and Coupling
- Page ID
- 357489
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)We have seen that for systems of coupled reactions, changing the concentration of one of the components in the system affects all the other components, even if they are not directly reacting with the one that is changed. We can use the same principles to explain why it is possible to carry out reactions that are thermodynamically unfavorable. We will consider a fairly simple example and then move on to see how this works in biological systems.
Many metals are not found in their elemental form. For example, copper—an important metal used for a wide range of applications from wires to roofs—is often found as chalcocite, an ore containing copper as copper sulfide. We can imagine a simple chemical reaction to separate the copper from the sulfide:[14] \[\mathrm{Cu}_{2} \mathrm{S}(s) \rightleftarrows 2 \mathrm{Cu}(s)+\mathrm{S}(s) \quad \Delta \mathrm{G}^{0}=86.2 \mathrm{kJ} / \mathrm{mol}\]
Note that this reaction is a redox reaction in which the \(\mathrm{Cu}^{+}\) ion is reduced to \(\mathrm{Cu}\) by the addition of an electron (from the sulfide \(\mathrm{S}^{2-}\), which is oxidized to sulfur with an oxidation state of 0.) Unfortunately, because the free energy change for this reaction is positive, the system at equilibrium is composed mostly of \(\mathrm{Cu}_{2} \mathrm{S}(s)\). How can we get copper out of copper sulfide? One possibility to exploit the reaction between sulfur and oxygen: \[\mathrm{S}(s)+\mathrm{O}_{2}(g) \rightleftarrows \mathrm{SO}_{2}(g), \text { for which } \Delta \mathrm{G}^{\circ}=-300.1 \mathrm{kJ} / \mathrm{mol}\]
This reaction is highly favorable and “goes” toward the production of \(\mathrm{SO}_{2}\)2. It is basically the burning of sulfur (analogous to the burning of carbon) and is another redox reaction in which the sulfur is oxidized (from an oxidation state of 0 to +4). Note that one reason why this reaction is so favorable is the formation of the strong \(\mathrm{S-O}\) bonds, which is a highly exothermic process.
If we take \(\mathrm{Cu}_{2}\mathrm{S}(s)\) together with \(\mathrm{O}_{2}(g)\), we have a system composed of two reactions: \[\begin{aligned}
&\mathrm{Cu}_{2} \mathrm{~S}(s) \rightleftarrows 2 \mathrm{Cu}(s)+\mathrm{S}(s) \text { [reaction 1] } \\
&\mathrm{S}(s)+\mathrm{O}_{2}(g) \rightleftarrows \mathrm{SO}_{2}(g) \text { [reaction 2] }
\end{aligned}\]
These two reactions share a common component (\(\mathrm{S}(s)\)); therefore, they are coupled. Imagine what happens when reaction 1 proceeds, even a little. The \(\mathrm{S}(s)\) produced can then react with the \(\mathrm{O}_{2}(g)\) present. As this reaction proceeds toward completion, \(\mathrm{S}(s)\) is removed, leaving \(\mathrm{Cu}(s)\) and \(\mathrm{SO}_{2} (g)\). Based on Le Chatelier’s principle, reaction 1 is now out of equilibrium, and thus generates more \(\mathrm{S}(s)\) and \(\mathrm{Cu}(s)\). Reaction 1 in isolation produces relatively little copper or sulfur, but it is dragged toward the products by reaction 2, a favorable reaction that removes sulfur from the system. If we assume that there are no other reactions occurring within the system, we can calculate the \(\Delta \mathrm{G}^{\circ}\) for the coupled reactions 1 and 2. Under standard conditions, we simply add the reactions together:
| \(\mathrm{Cu}_{2}\mathrm{S}(s) \rightleftarrows 2\mathrm{Cu}(s) + \mathrm{S}(s)\) | \(86.2 \mathrm{~kJ/mol}\) |
| \(\mathrm{S}(s) + \mathrm{O}_{2}(g) \rightleftarrows \mathrm{SO}_{2} (g)\) | \(-300.1 \mathrm{~kJ/mol}\) |
| \(\mathrm{Cu}_{2}\mathrm{S}(s) + \mathrm{O}_{2} (g) \rightleftarrows 2\mathrm{Cu}(s) + \mathrm{SO}_{2} (g)\) | \(-213.9 \mathrm{~kJ/mol}\) |
So, the \(\Delta \mathrm{G}^{\circ}\) for the coupled reaction is \(-213.9 \mathrm{~kJ/mol}\). This same basic logic applies to any coupled reaction system. Note that the common intermediate linking these two reactions is sulfur (\(\mathrm{S}\)). However, it is not always so simple to identify the common intermediate. In this system, we are tacitly assuming that \(\mathrm{O}_{2}\) and \(\mathrm{SO}_{2}\) do not react with either \(\mathrm{Cu}_{2}\mathrm{S}\) or \(\mathrm{SO}_{2}\). If they did, those reactions would also need to be considered in our analysis. In fact, we need to consider all of the reactions that are possible with a system. This is normally not a big issue with simple chemical systems that contain relatively small numbers of different types of molecules (sometimes called species), but it is a significant concern when we consider biological or ecological systems that contain thousands of different types of molecules, which can interact and react in a number of ways.
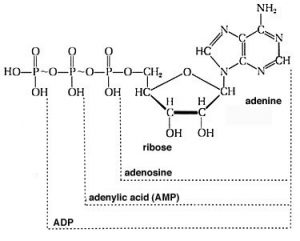
For example, you may have learned in biology that the molecule adenosine triphosphate (\(\mathrm{ATP}\)) is used to store and provide energy for cellular processes. What exactly does this mean? First, let us look at the structure of \(\mathrm{ATP}\): it is composed of a base called adenine, a sugar ribose, and three phosphate units. For our purposes, the adenine base and sugar (called adenosine when attached to each other) are irrelevant. They do not change during most of the reactions in which \(\mathrm{ATP}\) takes part. They are organic “building blocks” with functional groups that allow them to interact with other components in the cell for other functions (for example, in \(\mathrm{RNA}\) and \(\mathrm{DNA}\)). To examine energy transfer, we can just use “A” (adenosine) to stand in for their structure. The important bit for our purposes are the phosphates hooked together by the \(\mathrm{P—O—P}\) (phosphoanhydride) linkages. At physiological \(\mathrm{pH}\), most (if not all) of the oxygens of the phosphate esters are deprotonated. This means that there is a fairly high concentration of charge in this tri-ester side chain, which acts to destabilize it. The bonds holding it together are relatively weak, and the molecule reacts with any available entity to relieve some of this strain and form even more stable bonds. For example, \(\mathrm{ATP}\) is unstable in water and reacts (hydrolyzes) to form adenosine diphosphate (\(\mathrm{ADP}\)) and inorganic phosphate (\(\mathrm{HPO}_{4}\)), which is often written as \(\mathrm{P}_{i}\). This reaction is written as \(\mathrm{ATP} + \mathrm{H}_{2}\mathrm{O} \rightleftarrows \mathrm{ADP} + \mathrm{P}_{i}\).
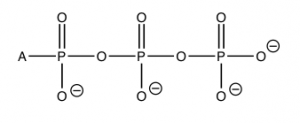
The standard free energy change for this reaction \(\Delta \mathrm{G}^{\circ} = – 29 \mathrm{~kJ/mol}\) (at \(\mathrm{pH } 7\)). This is a highly exergonic (heat or energy releasing) reaction; both the enthalpy and entropy changes for this reaction are favorable. \(\Delta \mathrm{H}\) is negative and \(\Delta \mathrm{S}\) is positive. It makes sense that the entropy change is positive. After all, we are producing two molecules from one. The enthalpy change also makes sense. We have already mentioned that \(\mathrm{ATP}\) is unstable, and the loss of one of the phosphate groups relieves some of the strain caused by the charge repulsion between the three negatively charged phosphate groups in \(\mathrm{ATP}\). The bond energies in the product are stronger than the bond energies in the reactants and thus the reaction is exothermic. Like everything in living systems, this is all somewhat complicated by the presence of other substances in the cellular fluids, such as the metal ions \(\mathrm{Ca}^{2+}\) and \(\mathrm{Mg}^{2+}\), and changes in pH. However, the explanation is still valid. Make sure that you do not fall prey to the commonly held misconception that it is the breaking of the \(\mathrm{P—O}\) bond that releases energy. On the contrary—it is the formation of more stable (stronger) bonds that releases energy.
If we go one step further and look at the actual free energy change \(\Delta \mathrm{G}\) (as opposed to the standard change), using typical cellular concentrations of \(\mathrm{ATP}\), \(\mathrm{ADP}\) and \(\mathrm{P}_{i}\), and \(\Delta \mathrm{G} = \Delta \mathrm{G}^{\circ} + \mathrm{RT} \ln \mathrm{Q}\) (where \(\mathrm{Q}=[\mathrm{ADP}]\left[\mathrm{P}_{i}\right] /[\mathrm{ATP}]\)), we can calculate: \(\Delta \mathrm{G} = – 52 \mathrm{~kJ/mol}\), assuming that the concentration of \(\mathrm{ATP}\) is typically about ten times that of \(\mathrm{ADP}\), and that \(\left[\mathrm{P}_{i}\right]\) is about \(0.001 \mathrm{~M}\). So in real conditions in the cell, the Gibbs free energy change is much higher than the standard Gibbs free energy change. This energy is not wasted; it is used to drive other reactions that would not otherwise occur. However, this energy cannot be used to drive just any random reaction. The reactions have to be coupled by common intermediates (just like the carbon dioxide carbonate system).
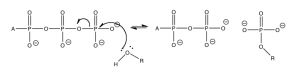
A typical reaction scenario is the transfer of the terminal phosphate group to another biomolecule as shown in the diagram. This transfer occurs with lipids and proteins, but typically the reacting group is an alcohol (\(\mathrm{ROH}\)) or sometimes a carboxylic acid (\(\mathrm{RCOOH}\)). The reaction that takes place is almost the same as the hydrolysis reaction except that the incoming nucleophile has much more “stuff” attached to the oxygen.

The formation of these phosphate esters makes the original functional group more reactive. For example, the formation of an amide bond (the major bond that holds proteins together) is normally exergonic (about \(+2\) to \(4 \mathrm{~kJ/mol}\)). The formation of amide bonds is not spontaneous (you might want to think about what this means for the amide bonds in the proteins that make up a good portion of you). Therefore, protein synthesis is coupled with \(\mathrm{ATP}\) hydrolysis, as is the production of many biomolecules, sugars, lipids, \(\mathrm{RNA}\), and \(\mathrm{DNA}\). The reactions are complex, but each of them is driven by a series of individual reactions linked by common intermediates.
Now you might be asking: if \(\mathrm{ATP}\) is so unstable, how does it get formed in the first place and how can it be found at such high concentrations? The short answer involves two ideas that we have encountered before: first, while \(\mathrm{ATP}\) is unstable (like wood in the presence of \(\mathrm{O}_{2}\)), its hydrolysis does involve overcoming an activation energy and so under physiological conditions, an enzyme that can catalyze and coupled the hydrolysis of \(\mathrm{ATP}\) to other reactions (an \(\mathrm{ATP}\)ase) is needed; second, \(\mathrm{ATP}\) is formed through coupled reactions that link the oxidation of molecules such as glucose or through the direct absorption of energy in the form of light (photosynthesis). When glucose reacts with oxygen it forms carbon dioxide and water: \[\mathrm{C}_{6} \mathrm{H}_{12} \mathrm{O}_{6}+6 \mathrm{O}_{2} \rightleftarrows 6 \mathrm{CO}_{2}+6 \mathrm{H}_{2} \mathrm{O}\]
with an overall standard free energy change \(\Delta \mathrm{G}^{\circ} = –2870 \mathrm{~kJ/mol}\). The reasons for this high negative free energy change are that \(\Delta \mathrm{S}^{\circ}\) is positive (why do you think this is?), and there is a large negative \(\Delta \mathrm{H}^{\circ}\) change. Remember that \(\Delta \mathrm{H}^{\circ}\) can be approximated by looking at the changes in bond energy from reactants to products. A major reason for this high enthalpy change is that the bond energies in carbon dioxide and water are very high (a \(\mathrm{C=O}\) bond takes \(805 \mathrm{~kJ/mol}\) to break, and an \(\mathrm{O-H}\) bond \(463 \mathrm{~kJ/mol}\)), and therefore when \(\mathrm{C=O}\) and \(\mathrm{O-H}\) bonds are formed a large amount of energy is released. When one mole of glucose is completely oxidized to \(\mathrm{CO}_{2}\) and \(\mathrm{H}_{2}\mathrm{O}\), the energy produced is harnessed to ultimately produce \(\sim 36\) moles of \(\mathrm{ATP}\) (from \(\mathrm{ADP}\) and \(\mathrm{P}_{i}\)).
The mechanism(s) involved in this process are complex (involving intervening ion gradients and rotating enzymes), but the basic principle remains: the reactions are coupled by common and often complex intermediate processes. This reaction coupling leads to networks of reactions. The synthesis and reaction of \(\mathrm{ATP}\) (and \(\mathrm{ADP}\)) is governed by the same principles that govern much simpler reactions. Whether or not \(\mathrm{ATP}\) or \(\mathrm{ADP}\) is the dominant species in any cellular compartment depends upon the conditions and what substrates are present to form a reaction.
Questions
Questions to Answer
- Can you draw the protonated form of \(\mathrm{ATP}\)?
- Can you draw the unprotonated form of \(\mathrm{ATP}\), showing how the negative charge is stabilized by the surrounding cellular fluids? (Hint: the fluid is mainly water.)
- The \(\mathrm{pK}_{a}\)'s of phosphoric acid (\(\mathrm{H}_{3}\mathrm{PO}_{4}\)) are \(2.15\), \(7.2\) and \(12.35\). Is the \(\mathrm{ATP}\) protonated or deprotonated in the cellular environment?
- Write out a hypothetical sequence of two reactions that result in the production of a thermodynamically unfavorable product.
- How can you tell whether two reactions are coupled?
- Why do biological systems rely on coupled reactions?
- If \(\mathrm{ATP}\) is unstable, how is it possible that \(\mathrm{ATP}\) can exist at high concentrations within the cell?
Questions to Ponder
- If you are trying to determine if two reactions are coupled, what do you look for?
- Coupling allows unfavorable reactions to occur. Why doesn’t this violate the laws of thermodynamics?Assume that you have a set of five coupled reactions. What factors could complicate the behavior of the system?
- How could you insure that an unfavorable reaction continued to occur at a significant (useful)
rate?


