6.2: Solubility: why do some things form solutions and others not?
- Page ID
- 355886
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Let us say you have a \(100-\mathrm{mL}\) graduated cylinder and you take \(50 \mathrm{~mL}\) of ethanol and add it to \(50 \mathrm{~mL}\) of water. You might be surprised to find that the volume of the resulting solution is less than \(100 \mathrm{~mL}\). In fact, it is about \(98 \mathrm{~mL}\), assuming good technique (no spilling). How can we explain this? Well, we can first reassure ourselves that matter has not been destroyed. If we weigh the solution, it weighs the same as \(50 \mathrm{~mL}\) of water plus \(50 \mathrm{~mL}\) of ethanol. This means that the density of the water–ethanol solution must be greater than the density of either the water or ethanol alone. At the molecular level, we can immediately deduce that the molecules are closer together in the ethanol and water mixture than they were when pure (before mixing) –try drawing a molecular level picture of this to convince yourself that this is possible. Now, if you took \(50 \mathrm{~mL}\) of oil and \(50 \mathrm{~mL}\) of water, you would find that they do not mix—no matter how hard you tried. They will always separate away from one another into two layers. What factors determine whether or not substances form solutions?
First, we need to be aware that solubility is not an all-or-nothing property. Even in the case of oil and water, a very small number of oil molecules are present in the water (the aqueous phase), and a small number of water molecules are present in the oil. There are a number of ways to describe solubility. The most common way is to define the number of moles of solute per liter of solution. This is called the solution’s molarity (\(\mathrm{M}, \mathrm{~mol/L}\)). Another common way is to define the number grams of solute per mass of solution. For example: \(1 \mathrm{~mg}\) (\(10^{-3} \mathrm{~g}\)) of solute dissolved in \(1 \mathrm{~kg}\) (\(10^{3} \mathrm{~g}\)) of solution is 1 part per million (\(10^{6}\)) solute, or \(1 \mathrm{~ppm}\). As you might expect, given the temperature term in the free energy equation, solubility data are always reported at a particular temperature. If no more solute can dissolve at a given temperature, the solution is said to be saturated; if more solute can dissolve, it is unsaturated.
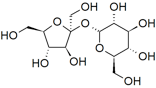
If we look at the structure of compounds that dissolve in water, we can begin to see some trends: hydrocarbons are not very soluble in water (remember from Chapter \(4\) that these are compounds composed only of carbon and hydrogen), whereas alcohols (hydrocarbons with an \(—\mathrm{O–H}\) group attached) with up to 3 carbons are completely soluble. As the number of carbon atoms increases, the solubility of the compound in water decreases. For example, hexanol (\(\mathrm{CH}_{3}\mathrm{CH}_{2}\mathrm{CH}_{2}\mathrm{CH}_{2}\mathrm{CH}_{2}\mathrm{CH}_{2}\mathrm{OH}\)), is only very slightly soluble in water (\(0.4 \mathrm{~g/L}\)). So perhaps the hydroxyl (\(—\mathrm{O–H}\)) group is responsible for the molecule’s solubility in water. Evidence supporting this hypothesis can be found in the fact that diols (compounds with \(2—\mathrm{O–H}\) groups) are more soluble than similar alcohols. For example, compared to hexanol, 1,6-hexanediol (\(\mathrm{HO}\mathrm{CH}_{2}\mathrm{CH}_{2}\mathrm{CH}_{2}\mathrm{CH}_{2}\mathrm{CH}_{2}\mathrm{CH}_{2}\mathrm{OH}\)) is quite soluble in water. More familiar water-soluble compounds such as the sugars glucose, fructose, and sucrose (a dimer of glucose and fructose – shown in the figure) are, in fact, polyalcohols. Each of their six carbons is attached to a hydroxyl group.
| Compound | Molar Mass (\mathrm{g/mol}\)) | Structure | Solubility (\(\mathrm{g/L}\)) \(20 { }^{\circ}\mathrm{C}\) |
| Propane | \(44\) | \(\(\mathrm{CH}_{3}\mathrm{CH}_{2}\mathrm{CH}_{3}\) | \(0.07 \mathrm{~g/L}\) |
| Ethanol | \(46\) | \(\mathrm{CH}_{3}\mathrm{CH}_{2}\mathrm{OH}\) | Completely miscible |
| Dimethyl ether | \(46\) | \(\(\mathrm{CH}_{3}\mathrm{OCH}_{3}\) | \(328 \mathrm{~g/L}\) |
| Pentane | \(72\) | \(\mathrm{CH}_{3}\mathrm{CH}_{2}\mathrm{CH}_{2}\mathrm{CH}_{2}\mathrm{CH}_{3}\) | \(0.4 \mathrm{~g/L}\) |
| Butanol | \(74\) | \(\mathrm{CH}_{3}\mathrm{CH}_{2}\mathrm{CH}_{2}\mathrm{CH}_{2}\mathrm{OH}\) | \(80 \mathrm{~g/L}\) |
| Diethyl ether | \(74\) | \(\mathrm{CH}_{3}\mathrm{CH}_{2}\mathrm{OCH}_{2}\mathrm{CH}_{3}\) | \(69 \mathrm{~g/L}\) |
| Hexanol | \(102\) | \(\mathrm{CH}_{3}\mathrm{CH}_{2}\mathrm{CH}_{2}\mathrm{CH}_{2}\mathrm{CH}_{2}\mathrm{CH}_{2}\mathrm{OH}\) | \(0.4 \mathrm{~g/L}\) |
| 1,6 Hexanediol | \(226\) | \(\mathrm{HO}\mathrm{CH}_{2}\mathrm{CH}_{2}\mathrm{CH}_{2}\mathrm{CH}_{2}\mathrm{CH}_{2}\mathrm{CH}_{2}\mathrm{OH}\) | \(500 \mathrm{~g/L}\) |
| Glucose | \(180\) | \(\mathrm{C}_{6}\mathrm{H}_{12}\mathrm{O}_{6}\) | \(910 \mathrm{~g/L}\) |
Questions
Questions to Answer
- Make a list of substances that you know dissolve in water.
- Which of these dissolve: metals, ionic compounds, molecular compounds (polar, non-polar), network solids (diamond graphite)?
- Can you make any generalizations about which things dissolve and which don’t?
- What must happen in order for something to dissolve in water?
- How would you design an experiment to determine the solubility of a solute?
- How would you determine whether or not a solution was saturated?
- Draw a molecular level picture of a solution of ethanol and water showing why the solution is more dense than the separate liquids.
- Draw a molecular level picture of an oil and water mixture.
- Draw a molecular level picture of the process of solution
- When you try mixing oil and water, which layer ends up on top? Why?
Question to Ponder
- You have a saturated solution, with some solid solute present.
- Do you think the solute particles that are in solution are the same ones over time?
- How would you determine whether they were the same?
Questions for Later
- What would you predict for the sign of \(\Delta \mathrm{S}\) upon the formation of any solution? Why?
- What would you predict for the sign of \(\Delta \mathrm{H}\) upon the formation of any solution? Why?
- What would you predict for the sign of \(\Delta \mathrm{G}\) upon the formation of any solution? Why?


