Electricity and Electrochemistry
- Page ID
- 54130
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Skills to Develop
- Distinguish between static and current electricity
- Describe the electrostatic force and Coulomb's Law
- Define ionic compounds, an electrolyte, and the components of an electrolyte
- Describe electrolysis and Berzelius' dualistic theory
The earliest studies of electricity focused on electrostatics. Static electricity can be produced when certain materials are rubbed together, like silk or hair on some metals or plastics (this is called the triboelectric effect). This leads to a separation of charge, with positive charge on the silk or hair and negative charge on the metal or plastic. You've probably noticed how this can lead to things sticking to each other if they have opposite charges, and you can also observe that the same charges will repel each other. Benjamin Franklin (one of the few early American scientists) proposed that the positive and negative charges resulted from having either too much or too little of the same "electrical fluid".
The electrostatic force had been studied by many scientists, some of whom suggested that it followed a law similar to Newton's law for gravity, but Coulomb gets credit for this, because he did many experiments that improved understanding. In 1785, he published the law in its current form. The equation is:
\[F=\frac{kQq}{r^{2}}\]
where F is the force, k is a constant, Q and q are two charges, and r is the distance between Q and Q. Like charges (+/+ or -/-) repel and opposite charges (+/-) attract.
Current electricity, like the electricity used in any electrical device today, was discovered a little later. Galvani was studying physiology, and noticed that the legs of dead frogs twitched when in contact with two different metals. He attributed this to "animal electricity." Volta did many more experiments and discovered that animals were unnecessary for the effect. He put a piece of paper soaked in saltwater (the electrolyte, because it conducts electricity) between disks of two different metals, then connected the metals with wire and noticed that electrical current flowed. When he stacked these on top of each other, alternating the metals, the effect increased. This device for generating current came to be called a "voltaic pile."
Volta published his pile reports in 1800. That same year, two English scientists published their results of splitting water into hydrogen and oxygen using a Voltaic pile. This is an example of electrolysis, which means using electricity to break chemical compounds.
Humphry Davy was a scientist and popular lecturer who had discovered "laughing gas" or nitrous oxide, which is still used by doctors and dentists. He did many electrochemical studies after 1800. He noticed that during electrolysis of various compounds, hydrogen, metals, or bases appear around the negative pole, and oxygen or acid appears around the positive pole. Based on this, he guessed he could use electricity to break chemical bonds. The strong bases KOH and NaOH (potassium hydroxide and sodium hydroxide) had been known for a long time. (A base neutralizes an acid.) Sodium and potassium were believed to be elements but had not been isolated. Davy was able to isolate them by melting and electrolyzing the solid sodium hydroxide and potassium hydroxide. The solid bases didn't conduct electricity, but once melted they did conduct, and then they separated into shiny metal and a gas.
K and Na are alkali metals. When Davy isolated them, he found that they were light, soft metals that react vigorously and spontaneously with water and air. So they have to be stored under oil. Davy also isolated magnesium, calcium, strontium, and barium (Mg, Ca, Sr, and Ba) using a slightly modified procedure involving mercury. These are the alkaline earth metals. They are also soft, light metals, but they are not quite as reactive as the alkali metals.
Berzelius was a very influential chemist who worked very hard and very carefully to determine atomic weights, without using Gay-Lussac's law or Avogadro's hypothesis, and whom we mentioned earlier because he introduced the idea of isomerism. He also did many electrochemical studies and took Davy's hypothesis, that chemical and electrical attraction are the same force, much farther. His dualistic theory said: elements have positive or negative polarity, and chemical reactions partially neutralize that polarity. For example:
Cu (positive) + O (negative) → CuO (approximately neutral)
He used the terms electropositive and electronegative, which are still used today with a somewhat different meaning. He thought oxygen was the most electronegative element, while metals were generally electropositive. He also considered the element polarity to be on a spectrum, so that sulfur, for instance, was positive with respect to oxygen and negative with respect to metals. Thus it could combine with both; this is very different from the modern understanding of Coulomb attraction because he did not have a clear sense of neutrality.
Ionic compounds are usually composed of a metal and a non-metal. For instance, salt, and many rocks, are ionic compounds. They are different from molecular compounds because they do not have distinct molecules that vaporize as a unit. They are held together by charges, not quite as Berzelius thought, but close enough that his theory worked all right for them.

However, Berzelius' theory did not work well for molecular compounds! He said Avogadro must be wrong because two oxygen (O) atoms would have the same polarity and thus repel each other. Now we know that chemical bonding is more complicated than Berzelius realized. O atoms don't repel each other, and do form a diatomic molecule.
Berzelius' theory delayed the progress of science for about 50 years because he didn't believe Avogadro's hypothesis, which was needed for the next big breakthrough in chemistry.
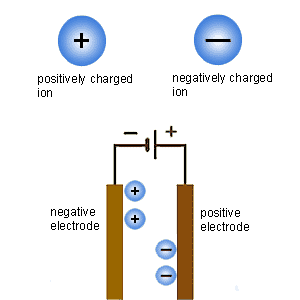
Faraday was born to a poor family, and discovered chemistry when working in a book-making shop, where we worked on Jane Marcet's Conversations on Chemistry (one of the most popular textbooks at the time, it is surprising that it was written by a woman). He became Davy's assistant, and became a great experimentalist. He helped invent the words used for electrochemistry, such as ion, anion, cation, electrolyte and electrolysis. At that time, anion and cation meant the parts of the electrolyte (the salt used to make water conductive) that appeared at each electrode. Faraday studied the amount of current necessary to produce an amount of an electrolysis product. For instance, the current that produced 1g of hydrogen produced 8g of oxygen, 36g of chlorine, 125g of iodine, 104g of lead or 58g of tin. Faraday called the O, Cl and I anions, meaning that they were produced at the positive pole, and Sn and Pb cations, meaning that they were produced at the negative pole. These numbers could have solved the atomic weights problem, just like Avogadro's hypothesis, but Faraday wasn't interested in atomic weights or theories, and Berzelius, who was, didn't believe Faraday's electrical laws, just like he didn't believe Avogadro's hypothesis.
Outside Link
Contributors and Attributions
Emily V Eames (City College of San Francisco)

