6.3: Rearangement
- Page ID
- 379746
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Heptane
The mass spectrum of heptane is shown in Figure \(\PageIndex{1}\). This mass spectrum is consistent with the fragmentation patterns discussed in the previous section. The molecular ion, C7H16•+ is observed at 100 m/z and a series of cleavage peaks are observed for loss of CH3• (M - 15), C2H5• (M - 29), and C3H7• (M - 43). These peaks are observed at 85 m/z , 71 m/z , and 57 m/z respectively. This fragmentation is characteristic for a linear hydrocarbon.

McLafferty Rearrangement
Some functional groups, however, can undergo very different fragmentation processes than the direct cleavage discussed so far. One common example is the McLafferty rearrangement (Figure \(\PageIndex{2}\)) which results in formation of an intact neutral molecule and a radical ion, both with an even mass to charge ratio. Since the most intense direct cleavage fragments have odd mass to charge ratios, this fragmentation pattern is very useful for identifying carbonyl compounds and for determining their structure. The McLafferty rearrangement is often observed for carbonyl compounds that contain a linear alkyl chain. If this alkyl chain is long enough, a six-membered ring forms from the carbonyl oxygen to the hydrogen on the fourth carbon. This spacing allows the hydrogen to transfer to the carbonyl oxygen via a six membered ring. This is followed by a rearrangement of the electrons to break the beta C-C bond, the second bond from the carbonyl carbon, to form an alkene and resonance stabilized radical with the carbonyl group. The McLafferty rearrangement is energetically favorable because it results in loss of a neutral alkene and formation of a resonance stabilized radical. Both these fragments may be observed in the mass spectrum, depending upon which fragment retains the charge. Figure \(\PageIndex{2}\) shows the charge on the resonance stabilized radical, this is the McLafferty ion. The alkene is referred to as the McLafferty compliment.

The products from the McLafferty rearrangement are observed in the mass spectra of C-7 carbonyl compounds shown in Figures 22-26. Draw structures for the following compounds and use the mechanism shown in Figure 21 to predict the mass of the two fragments formed by the McLafferty rearrangement. Then compare these predictions with the mass spectra shown in Figures \(\PageIndex{3}\) - \(\PageIndex{7}\).
- heptanal
- 2-heptanone
- 3-heptanone
- 4-heptanone
- heptanoic acid
Heptanal
The mass spectrum of heptanal shown in Figure \(\PageIndex{3}\) contains two even mass ions. C2H4O+ m/z 44 is produced by the McLafferty rearrangement of an aldehyde and is a characteristic peak that is very useful for interpretation of aldehydes. The McLafferty compliment, C5H10+, is observed at 70 m/z . The McLafferty compliment is produced when the charge is transferred to the alkene fragment during the rearrangement.

2-Heptanone
The mass spectrum of 2-hepanone shown in Figure \(\PageIndex{4}\) is easily distinguished from heptanal because the McLafferty rearrangement breaks the C-C bond between C-3 and C-4. This results in loss C4H8 to give the McLafferty ion for a 2-ketone, C3H6O+, at 58 m/z. The McLafferty compliment, C4H8+ (56 m/z) is not observed for 2-heptanone.
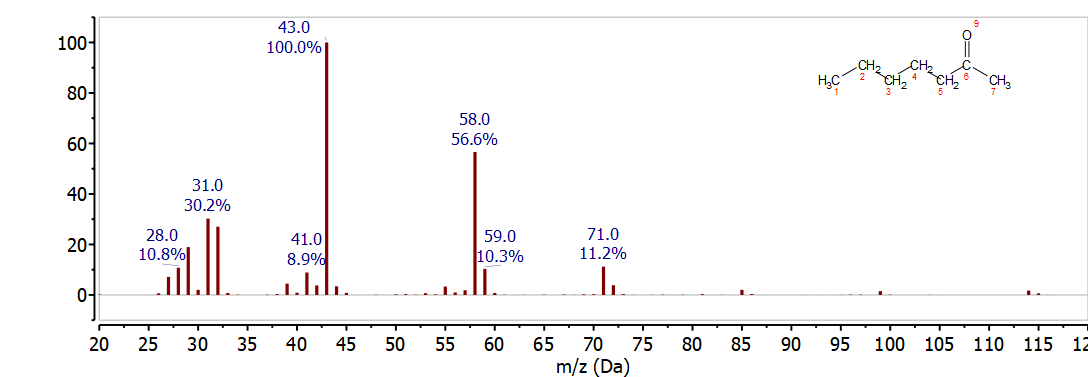
3-Heptanone
The mass spectrum of 3-hepanone in Figure \(\PageIndex{5}\) is easily distinguished from heptanal and 2-heptanone because the McLafferty rearrangement breaks the C-C bond between C-4 and C-5. This results in loss C3H6 to give the McLafferty ion for a 3-ketone, C4H8O+, at 72 m/z. The McLafferty compliment, C3H6+ (42 m/z) is not observed for 3-heptanone.

4-Heptanone
The mass spectrum of 4-hepanone shown in Figure \(\PageIndex{6}\) is easily distinguished from heptanal, 2-heptanone, and 3-heptanone. The McLafferty rearrangement would break the C-C bond between C-2 and C-3. This results in loss C2H4 to give the McLafferty ion for a 4-ketone, C5H10O+, at 86 m/z - which has a very low intensity in the mass spectrum of 4-heptanone shown in figure 25. The two major peaks in this spectrum 43 m/z and 71 m/z correspond to alpha cleavage to produce C3H7 and C4H7O which would be observed at 43 m/z and 71 m/z respectively. In this molecule the direct cleavage is highly favored over the McLafferty rearrangement. In this case the faster kinetics of the direct cleavage are favored over the concerted mechanism required for the rearrangement.
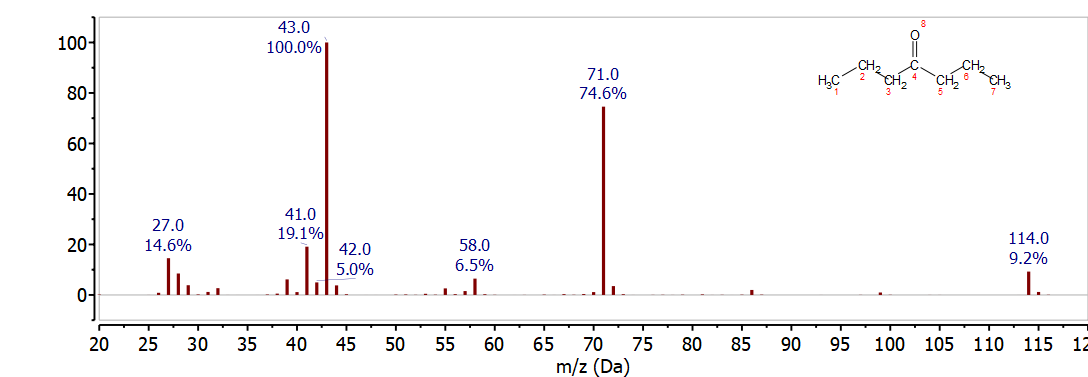
Heptanoic Acid
The mass spectrum of heptanoic acid shown in Figure \(\PageIndex{7}\) is easily distinguished from heptanal, 2-heptanone, 3-heptanone, and 4-heptanone because the McLafferty rearrangement produces C2H4O2+ observed at 60 m/z and characteristic of a carboxylic acid. In this case the McLafferty compliment, C5H8+, is not observed in the mass spectrum.

Based up on the discussion so far you should be able to identify many of the other fragments in these three mass spectra. Spend some time with a piece of scratch paper and see what you come up with.


