6.1: Molecular Ion
- Page ID
- 374827
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Molecular Ion
The molecular ion provides the molecular mass of the analyte and is the first clue used to interpret a mass spectrum. The mass to charge ratio of the molecular ion is based up on the mass of the most abundant isotope for each element in the molecule. This is not the relative atomic mass from the periodic table. Since many mass spectrometers have unit mass resolution, the isotope mass is normally rounded to the nearest whole number, this is called the nominal mass. For example the molecular ion for CHBr3 is observed at 250 m/z;(12 + 1 + 3 \(\times\) 79)=250), not at the relative molecular weight of 253. The mass of the molecular ion is based upon the mass of the isotope with the highest natural abundance. The most common bromine isotope is 79Br. Do not use the weighted average atomic weight for Br (79.9) which is based upon the natural abundance of 79Br and 81Br. The mass spectrum of CHBr3 includes ions for all the naturally occurring isotopes and the intensity of each peak depends upon the probability for that combination of isotopes. These patterns are discussed in detail in the section on isotope abundance.
In many mass spectra, the molecular ion is easily identified as the ion with the highest mass to charge ratio. However, this assignment should be made with caution because the highest mass to charge ion may be an impurity, an isotope of the molecular ion, or a fragment. Many compounds fragment easily and no molecular ion is observed in the 70 eV EI spectrum. It is important to clarify that the molecular ion IS NOT necessarily the ion with the greatest abundance, the ion with the greatest abundance is called the base peak. The base peak is the peak with the greatest abundance. The mass spectrum is usually normalized so that this peak has an intensity of 100.
A list of molecular ion characteristics are in Table \(\PageIndex{1}\) to help you identify them in a mass spectrum. Low energy EI, where the ionization energy is reduced, often increases in intensity of the molecular ion. Chemical Ionization, CI, is also useful for identifying the molecular ion since the the adduct ion is often more stable than the radical cation produced by electron ionization. The adduct ion is often formed by protonating the analyte to form \((\mathrm{M}+\mathrm{H})\) and is observed at a mass to charge ratio of M+1.
| The mass to charge ratio must correspond to a reasonable molecular formula with the proper isotope abundance. |
| Most compounds have an even molecular mass. The one common exception to this is the "Nitrogen Rule" discussed below. |
| The Nitrogen Rule: Any compound with an odd number of nitrogen atoms will have an odd molecular mass. Any compound with an even number of nitrogen atoms (including zero) will have an even molecular mass. This is because nitrogen is the only common atom where the most common isotope has an odd valence and an even mass. For example: the molecular ion for CH4 is observed at 16 m/z, the molecular ion for NH3 is observed at 17 m/z, and the molecular ion for N2H4 is observed at 32 m/z. |
| If a peak is the molecular ion, the next highest mass fragment must correspond to the loss of a possible neutral fragment. For example, a peak that corresponds to loss of 5 u from the molecular ion is highly unlikely |
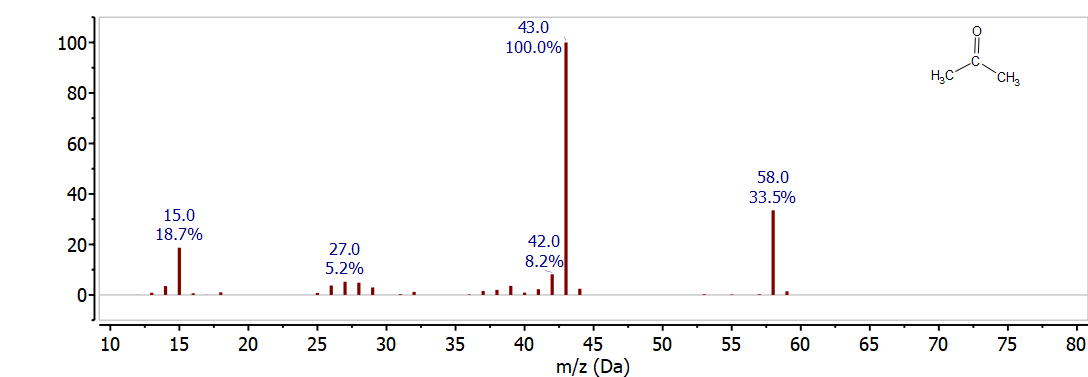
Figure \(\PageIndex{1}\) shows the mass spectrum of acetone (CH3COCH3). The molecular ion is clearly shown at 58 m/z (12 x 3 + 6 x 1 + 16 = 58). The base peak is at 43 m/z and corresponds to loss of 15 m/z from the intact molecule, this is caused by breaking a C-C bond for loss of a CH3• radical to give CH3CO+ at 43 m/z (12 x 2 + 3 x 1 + 16 = 43). The mass spectrum also includes several other minor peaks - the peak at 59 m/z is caused by the small abundance of C-13 that gives a small fraction of the acetone molecules a mass of 59; the peak at 15 m/z is caused by the CH3 fragment retaining the charge when the C-C bond breaks. These fragmentation and isotope patterns are discussed in more detail in the following sections.