3.5: Fast Atom Bombardment and Secondary Ion Mass Spectrometry
- Page ID
- 374795
Fast Atom Bombardment and Secondary Ion Mass Spectrometry. (1)
Fast Atom Bombardment (FAB) and Secondary Ion Mass Spectrometry (SIMS) both use high energy atoms to sputter and ionize the sample in a single step. In these techniques, a beam of rare gas neutrals (FAB) or ions (SIMS) is focused on the liquid or solid sample. The impact of this high energy beam causes the analyte molecules to sputter into the gas phase and ionize in a single step (Figure \(\PageIndex{1}\): Fast Atom Bombardment Source. ). The exact mechanism of this process is not well understood, but these techniques work well for compounds with molecular weights up to a few thousand dalton. Since no heating is required, sputtering techniques (especially FAB) are useful for study ing thermally labile compounds that decompose in conventional inlets (2, 3).
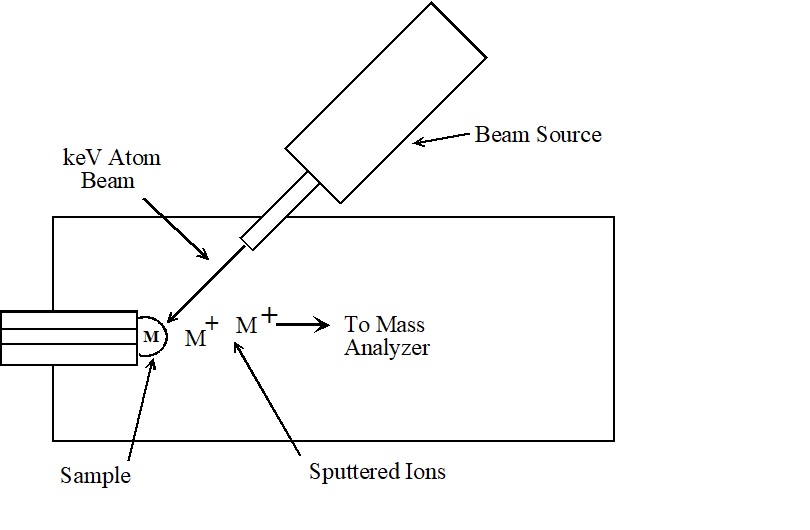
The most significant difference between FAB and SIMS is the sample preparation. In FAB the analyte is dissolved in a liquid matrix. A drop of the sample/matrix mixture is placed at the end of an insertion probe and introduced to the source region. The fast atom beam is focused on this droplet to produce analyte ions. Glycerol or similar low vapor pressure liquids are typically used for the matrix. Ideally, the analyte is soluble in the liquid matrix and a monolayer of analyte forms on the surface of the droplet. According to one theory, this monolayer concentrates the analyte while the dissolved sample provides a reservoir to replenish the monolayer as the analyte is depleted. Without this constant replenishment from the bulk solution, the ionizing beam will rapidly deplete the analyte and the signal is difficult to observe.
SIMS experiments(4) are used to study surface species and solid samples. Liquid SIMS (LSIMS) is very similar to FAB except cesium ions are used for higher energy collisions. No matrix is used and the ionizing beam is focused directly on the sample. Although this makes sampling more difficult, it is useful for studying surface chemistry. High resolution chemical maps are produced by scanning a tightly focused ionizing beam across the surface and depth profiles are produced by probing a single location(5,6). Although SIMS is a very sensitive and powerful technique for surface chemistry and materials analysis, the results are often difficult to quantitate.
References
- Barber, M.; Bordoli, R.S.; Elliott, G.J.; Sedgwick, R.D.; Tyler, A.N. Anal. Chem. 1982, 54, 645A-657A.
- Fenselau, C. Anal. Chem. 1982, 54, 105A-114A.
- Biemann, K. Anal. Chem. 1986, 58, 1288A-1300A.
- Day, R.J.; Unger, S.e.; Cooks, R.G. Anal. Chem. 1980, 82, 557A-572A.
- Winograd, N. Anal. Chem. 1993, 65, 622A-629A.
- Benninghoven, A.; Hagenhoff, B.; Niehuis, E. Anal. Chem. 1993, 65, 630A-640A.


