3.4: Matrix Assisted Laser Desorption/Ionization
- Page ID
- 374813
Matrix Assisted Laser Desorption/Ionization. (1,2)
Matrix Assisted Laser Desorption/Ionization (MALDI) is used to analyze extremely large molecules. This technique directly ionizes and vaporizes the analyte from the condensed phase. MALDI is often used for the analysis of synthetic and natural polymers, proteins, and peptides. Analysis of compounds with molecular weights up to 200,000 dalton is possible and this high mass limit is continually increasing.
In MALDI, both desorption and ionization are induced by a single laser pulse (Figure \(\PageIndex{1}\) ). The sample is prepared by mixing the analyte and a matrix compound chosen to absorb the laser wavelength. This is placed on a probe tip and dried. A vacuum lock is used to insert the probe into the source region of the mass spectrometer. A laser beam is then focused on this dried mixture and the energy from a laser pulse is absorbed by the matrix. This energy ejects analyte ions from the surface so that a mass spectrum is acquired for each laser pulse. The mechanism for this process is not well understood and is the subject of much controversy in the literature. This technique is more universal (works with more compounds) than other laser ionization techniques because the matrix absorbs the laser pulse. With other laser ionization techniques, the analyte must absorb at the laser wavelength. Typical MALDI spectra include the molecular ion, some multiply charged ions, and very few fragments.
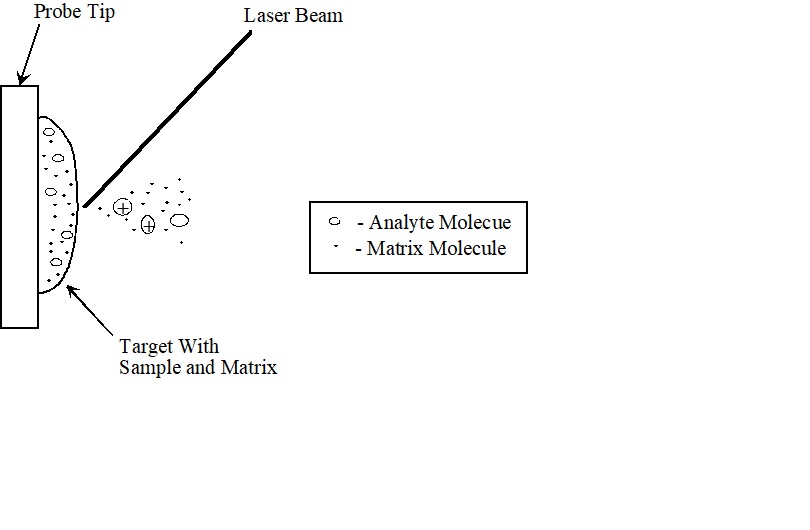
Figure \(\PageIndex{1}\) : MALDI Ionization Target with Sample and Matrix
Other Ionization Methods. There are several other ionization methods used for mass spectrometry and interested readers are referred to the chemical literature for additional information about other techniques. Field Desorption (3) was used for ionization and vaporization of moderate sized molecules before the development of FAB, electrospray, and MALDI. It is still an important technique for some analysis and is typically used for non-polar polymers and petroleum samples. Plasma Desorption (PD) (4, 5) is a technique used to analyze high molecular weight compounds before the development of MALDI and electrospray. However, it is very complex and has not found widespread application. Resonance Ionization Mass Spectrometry (RIMS) is used for selective atomic and molecular ionization. (6) Photoionization with lasers, lamps, and synchrotron sources is used to study the photochemistry and energetics of many compounds. (7) Lasers are used to ionize surface samples with Laser Microprobe Mass Analysis (LAMMA). (8, 9)
References
- Karas, M.; Hillenkamp, F. Anal. Chem. 1988, 60, 2299-2301.
- Fenselau, C. Anal. Chem. 1997, 69, 661A-665A.
- Lattimer, R.P.; Schulten, H.R. Anal. Chem. 1989, 61, 1201A-1215A.
- Macfarlane, R.D. Anal. Chem. 1983, 55, 1247A-1264A.
- Cotter, R.J. Anal. Chem. 1988, 60, 781A-793A.
- Young, J.P.; Shaw, R.W.; Smith, D.H. Anal. Chem. 1989, 61, 1271A-1279A.
- Syage Anal. Chem. 1990, 62, 505A.
- Van Grieken, R.; Adams, F.; Natusch, D. Anal. Chem. 1982, 54, 26A-41A.
- Hercules, D.M.; Day, R.J.; Balasanmugam, K.; Dang, T.A.; Li, C.P. Anal. Chem. 1982, 54, 280A-305A.


