Foundations for Nuclear Medicine
- Page ID
- 50767
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Nuclear medicine is a branch of medicine that uses radioactive isotopes (radionuclides) and relies in part on the decay of the atomic nucleus for diagnosis and treatment of disease. But nuclear medicine also relies on our ability to synthesize biomolecules that contain radioactive nuclei. The biomolecules behave exactly the same, whether they contain radioactive or non-radioactive nuclei. How can this be?
The biological activity of a molecule depends entirely on it's electronic structure, which determines its shape and size. The nucleus is buried inside the electrons, and occupies less than one millionth of a millionth of a percent of the atom's volume! By analogy, if the electrons moved around an area the size of a football field, the nucleus would be smaller than a marble in most atoms. It makes sense that some nuclear properties can be changed without altering the biological properties. To understand the relationship of the nucleus to the entire atom, let's look at the historical development of our modern picture of the atom. Along the way, we'll see how X-rays for medical use originate outside the nucleus by entirely different mechanisms.
Ernest Rutherford
The results of Thomson’s and other experiments implied that electrons were constituents of all matter and hence of all atoms. Since macroscopic samples of the elements are found to be electrically neutral, this meant that each atom probably contained a positively charged portion to balance the negative charge of its electrons. In an attempt to learn more about how positive and negative charges were distributed in atoms, Ernest Rutherford (1871 to 1937) and his coworkers performed numerous experiments in which α particles emitted from a radioactive element such as polonium were allowed to strike thin sheets of metals such as gold or platinum.

Figure \(\PageIndex{1}\) Schematic diagram of deflection of α particles by thin metal foil.
It was already known that the α particles carried a positive charge and traveled rapidly through gases in straight lines. Rutherford reasoned that in a solid, where the atoms were packed tightly together, there would be numerous collisions of α particles with electrons or with the unknown positive portions of the atoms. Since the mass of an individual electron was quite small, a great many collisions would be necessary to deflect an α particle from its original path, and Rutherford’s preliminary calculations indicated that most would go right through the metal targets or be deflected very little by the electrons. In 1909, confirmation of this expected result was entrusted to Hans Geiger and a young student, Ernest Marsden, who was working on his first research project.
The results of Geiger and Marsden’s work (using apparatus whose design is shown schematically in Fig. \(\PageIndex{1}\) were quite striking, as can be seen in YouTube videos or animations. Most of the α particles went straight through the sample or were deflected very little. These were observed by means of continuous luminescence of the ZnS screen at position 1 in the diagram. Observations made at greater angles from the initial path of the a particles (positions 2 and 3) revealed fewer and fewer flashes of light, but even at an angle nearly 180° from the initial path (position 4) a few α particles were detected coming backward from the target. This result amazed Rutherford. In his own words, “It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you. On consideration, I realized that this scattering backwards must be the result of a single collision, and when I made calculations I saw that it was impossible to get anything of that order of magnitude unless you took a system in which the greater part of the mass of an atom was concentrated in a minute nucleus”[1]. Rutherford’s interpretation of Geiger and Marsden’s experiment is shown schematically in Fig \(\PageIndex{2}\).
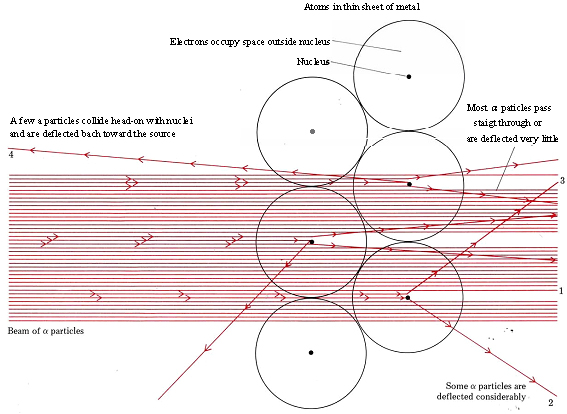
Quantitative calculations using these experimental results showed that the diameter of the nucleus was about one ten-thousandth that of the atom. The positive charge on the nucleus was found to be + Ze, where Z is the atomic number which indicates the number of protons, and is used to place each element in the modern periodic table. (For example, H is the first element and has Z = 1. He is the second element and Z = 2. The twentieth element in the valence table constructed earlier is Ca, and the nucleus of each Ca atom therefore has a charge of + 20e = 20 × 1.60 × 10–19 C = 32.0 × 10–19 C.) In order for an atom to remain electrically neutral, it must have a total of Z electrons outside the nucleus. These provide a charge of –Ze to balance the positive nuclear charge.
Wilhelm Roentgen and X-Rays
Wilhelm Roentgen (1845 to 1923) discovered earlier that if high voltages are applied to a cathode ray tube, rays were given off which could penetrate black paper or other materials opaque to visible light. He called this unusual radiation x-rays, the x indicating unknown. X-rays are produced when inner orbiting electrons on atoms of the "Target" (right electrode in figure) are struck by cathode rays (emitted by the cathode on the left in the figure), then the electrons are pulled back by the nucleus, releasing X-rays(which exit the tube downward in the figure). X-rays are high frequency electromagnetic radiation, like UV light, except higher in energy.
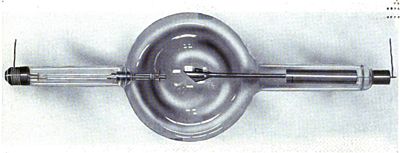
A historical documentary of medical X-ray tubes is | available.
Moseley and the Nucleus
The significance of the atomic number was firmly established in 1914 when H. G. Moseley (1888 to 1915) published the results of experiments in which he bombarded a large number of different metallic elements with electrons in a cathode-ray tube. Moseley found that the frequency of the x-rays was unique for each different metal. It depended on the atomic number--the number of protons-- (but not on the atomic weight) of the metal. (If you are not familiar with electromagnetic radiation or the term frequency, read The Nature of Electromagnetic Radiation where they are discussed more fully.) Using his x-ray frequencies, Moseley was able to establish the correct ordering in the periodic table for elements such as Co and Ni whose atomic weights disagreed with the positions to which Mendeleev had assigned them. His work confirmed the validity of Mendeleev’s assumption that chemical properties were more important than atomic weights.
From ChemPRIME: 4.9: The Nucleus
References
Contributors and Attributions
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.


