2: Nutrasweet: Physical-Chemical Concepts
- Page ID
- 40237
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Introduction
This section of the NutraSweet ChemCase could easily have been Section 1, a first section to present basic information about NutraSweet (aspartame). We include a brief review of physical and chemical considerations applied specifically to NutraSweet. You can find more detailed information presented in a broader context in a general chemistry text such as Atkins and Jones, Chemistry: Molecules, Matter, and Change (WH Freeman, NY, 1998).
Physical Properties
- All substances have properties that are described as physical, as they represent traits or properties of the chemical that are observed or measured without change in electronic structure or chemical nature.
- All substances have properties that are described as chemical; these do involve changes in electronic or chemical properties.
For example, a substance will have physical characteristics such as state of matter, boiling point, melting point, density, dipole moment, and molecular or atomic weight. Chemicals will also show chemical properties where electrons are gained or lost and bonds are broken and formed with, perhaps, atoms being lost, gained, rearranged, or replaced. A property such as solubility is often listed as physical, yet many examples of one compound dissolving in another involve a rearrangement of electron density of some type.
NutraSweet’s physical properties are important aspects of its value as a commercial product. Consideration of its chemical properties blends into its biochemical properties when assessment is made of its use as a sweetener.
If one were to introduce a sweetener like NutraSweet to the marketplace, what physical characteristics would be desirable?
- Convenient techniques are available to handle gases, solids, and liquids, but solids are especially easy to handle. NutraSweet is a solid.
- It is critical that a sweetener mix conveniently with the bulk material it is to sweeten. To mix a powdery solid with a powdery solid is pretty easy, so NutraSweet naturally mixes well with other solids. Packaged lemonade mixes or pouches of Equal bear witness to this physical characteristic.
- More significantly for NutraSweet is its solubility in water, so that solutions of the sweetener can be utilized. NutraSweet is soluble in water to a modest degree. But its intense sweetness requires only a small amount in solution to deliver the desired effect. So this physical property is only a modest limitation to aspartame’s use in soft drinks and reduced-calorie fruit juices, for example.
Chemical Properties
There are many, many chemical products whose properties are not determined by potential human contact or consumption. But when chemicals are developed for human consumption, an entirely new level of understanding of biochemical properties is required before the chemical can enter the marketplace.
General chemistry texts generally discuss chemistry in the absence of specific applications, although occasional problems or case studies (sidebars) may devote some space to a specific application. A principal purpose of this ChemCases study unit is to attempt to relate some of what is learned in introductory chemistry to a "real" situation, in this case the discovery, development, and marketing of a specific chemical substance, aspartame. More challenging considerations about aspartame lie with its chemical properties.
TYPES OF BONDS
Bonds between atoms can be classified as covalent, polar covalent, or ionic. If two atoms of the same or approximately the same electronegativity are bonded together, the electrons are held more or less equally between the two atoms and the bond is called covalent. The single bond in H-H and the double bond in H2C=CH2 are perfectly covalent because the attached atoms or groups (H and CH2) are identical in electronegativity. Carbon and hydrogen have about the same electronegativity, so bonds between these two atoms are generally called covalent. If we bond two atoms whose attraction for the electron pair differs considerably, such as H and Cl, then the electrons are more apt to be closer to the more electronegative atom, and that atom will assume a partial negative charge. As a result, the other atom is slightly electron deficient and will assume a slightly positive character. Such electron distortion results in a polar covalent bond (with electrons unequally shared) and is frequently represented as, say, Hd +-Cld - to reflect the polarity. Water is another common example of a polar covalent molecule Hd +-Od --Hd +.
It is important to appreciate the difference between a polar bond and a polar molecule. A molecule will be polar if the individual bond polarities are not cancelled by the molecular shape. For example Od -=Cd +=Od - has polar bonds, but its linearity results in a net dipole of zero. Water is not linear but tetrahedral, and the individual bond polarities are not internally cancelled. Similarly, though Hd +-Cld - is linear it is yet polar, for there will be a net dipole with only the two atoms. Carbon tetrachloride (CCl4), however, though it has polar C-Cl bonds, is a nonpolar molecule for its tetrahedral structure allows all the individual C-Cl bonds’ polarity to be cancelled (offset) by the shape.
[Q. Other examples]
A third type of bond, the ionic bond, occurs when an electron pair is formally transferred from one atom to another. Sodium chloride, Na+Cl- , is ionic because the "shared" electron pair is considered completely associated with the chlorine, so that the Cl is negative, a chloride ion (Cl-), and sodium is positive, a sodium ion (Na+).
In reality, the three bond types are somewhat arbitrary locations on a continuum from being shared equally (covalent) through being shared unequally (polar covalent) to being transferred (ionic).
Aspartame (Figure 1) has all three types of bonding in its chemical structure. The C-C and C-H bonds are close to covalent, so exhibit no real polarity. The C=O and C-O bonds exhibit polar covalent character, as do the C-N and N-H bonds. The N-H polarity is greater than the modest polarity shown by the other three. Aspartame contains a weakly basic –NH2 group (amine) and is typical of most "amino acids" in that it transfers the proton from the acid part of the molecule to the unshared pair of electrons of the amine forming –COO- and –NH3+. This does not happen in all the molecules. So some exist in this ionic form and some in the polar form with the polar covalent N-H and O-H bonds. The various types of bond polarities in aspartame profoundly affect the physical and chemical properties exhibited by the compound. Ionic compounds tend to have high melting and boiling points, for example, and aspartame’s melting point is 248-250oC, very high for carbon compounds of similar molecular formula but no ionic bonds.
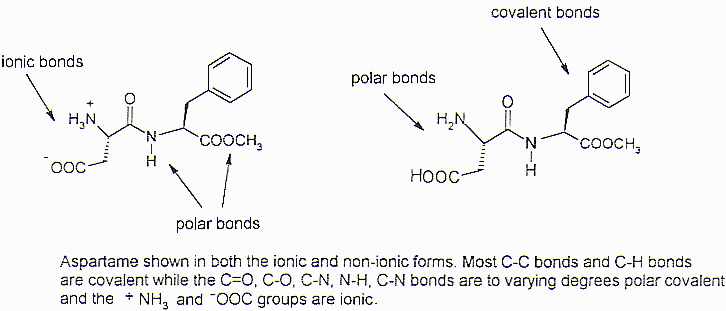
<>Figure 1: Aspartame
Melting is the property of "breaking" the forces that hold molecules together in the solid state, so that the greater molecular mobility of the liquid state is allowed. (Melting does not break bonds within a molecule, just between them.) It is not surprising that aspartame’s molecules are difficult to separate from each other, since there would be strong interaction between the positive –NH3+ group of one molecule and the –COO- of another molecule, for example.
Solubility is the only other physical property of aspartame that will be reviewed. Like melting point, it is closely related to the bonding characteristics.
One might argue that when one compound dissolves in another, two equals three (or more). Compound A, appearing in a smaller amount and thus called a solute, will need to interact with compound B, in the larger quantity and thus called a solvent, to effect solution. Compound A’s properties and structure may be changed modestly as when water dissolves in alcohol, or may be changed drastically, when solid sodium chloride dissolves in water to produce highly solvated (hydrated) sodium and chloride ions. Conversely, by interacting with the solute, solvent B's structure and properties may be changed little or considerably. A’s and/or B’s properties may change, but there is also a new entity, the solution, which has a life of its own, so to speak, with its own set of physical properties. Two, A and B, equals three, A, B, and the solution.
So when aspartame is dissolved in water, the molecular structure has changed from a solid form, with whatever forces were holding the molecules together, to a liquid solution, in which the aspartame is now interacting with water molecules. An understanding of the forces that hold molecules together in the solid state (i.e., ionic, Van de Waals, and dipole) and those that allow the solid to have its molecules separated by solvent molecules is useful. The terms hydrophilic and hydrophobic are often used to describe a molecule’s, or portions of a molecule’s, affinity for water, a polar substance. Hydrophilic means "water loving", suggesting an attraction to water and hence inferring some polarity. Conversely, hydrophobic, "water hating", suggests a lack of interest in associating with water, inferring a nonpolar character.
Water molecules are held together by polar forces called hydrogen bonds. Individually, they are fairly weak associations, but collectively, as many molecules form a network of hydrogen-bonded molecules (Figure 2).

Figure 2
Aspartame, as just mentioned, has ionic, polar covalent, and covalent bonding. The most important attractions between molecules are those associated with ionic interactions and polar covalent associations. Figure 3 suggests some possibilities. Why, and how, does aspartame dissolve in water, and why is it sparingly? Let’s investigate an answer in a strictly qualitative manner.

For aspartame to effectively dissolve in water, the forces that hold the aspartame molecules together must be broken, the forces that hold the water molecules together must be disrupted, and new forces between aspartame and water molecules must be established. These three aspects of solution making must occur in an energetically favorable manner or the entire process will not occur or not to any great extent.
Since it takes quite a bit of energy to separate aspartame molecules from each other, because of the polar and ionic interactions, and a modest amount of energy to disturb the hydrogen bonding network of water, there will need to be fairly extensive associations between aspartame and water for solution to occur. Such associations are indeed reasonable and expected since the d + hydrogen dipole of water can associate with the –COO- of the ionic aspartame molecules or the d - of the C=Os or the unshared pair of electrons (nonbonded electrons) on the oxygens or unprotonated nitrogens. The d - of the oxygen dipole of water can favorably associate to stabilize the –NH3+ portion of the ionic aspartame or the polarized d + H of the N-H bonds. Though strong intermolecular forces require breaking, they are replaced by quite favorable intermolecular associations between solute and solvent. That aspartame isn’t more soluble than about 3 g in 100 mL of water is due to the relatively large nonpolar ring portion of aspartame and other C-H and C-C bonds that are quite nonpolar and hence quite hydrophilic. Aspartame’s water solubility is pH dependent, not surprising for a compound with such polar functional groups.
Chemical and Biochemical Reactions
One significant chemical task is synthesis, the ability to make one chemical substance from another. Synthesis is a hallmark of what makes chemistry a marvelous and compelling science. Synthesis requires converting one substance to another by one or several steps. Each step is a separate chemical reaction. In this aspartame segment, some general concepts of reactions will be mentioned. Chapter 3 in Atkins and Jones introduces the topic.
With refreshing regularity, our bodies act as a magnificent synthetic factory, with thousands of chemical reactions occurring simultaneously. Foodstuffs are chemically and enzymatically degraded to produce new raw materials. These new compounds and the energy the body produces by oxidizing glucose are needed to manufacture the DNA, RNA, proteins, lipids, hormones, and the like that allow us to function as we do.
Of course, analogous processes occur throughout the natural world in insects, plants, and all other living organisms. One of science’s overriding challenges is to better understand such processes and to use that understanding in a prudent fashion.
Many types of chemical and biochemical reactions can occur. Four are indicated below.
| Substitution A-B + S ® A-S + B |
|
Addition A + B ® A-B |
| Elimination A-B ® A + B |
| Condensation A + B ® C + small molecule |
In substitution, a reagent (S) will react with a molecule (A-B) substituting itself in place of a part of the molecule (B in this example). In an addition reaction, two reactants (A and B) will combine to give one (A-B) with no other product. In elimination, a single molecule (A-B) will break apart into two molecules (A and B). Addition and elimination are the opposites of each other. In this example of condensation, two reactant molecules (A and B) combine to form a product molecule (C) and a small molecule, such as water.
If we look at the aspartame molecule, we see many bonds. An experienced chemist notes that there are two bonds that are easily made from readily available chemicals (Figure 4)

and that aspartame might then be synthesized by somehow reacting these chemicals together. Both reactions are condensations, with water being the small molecule exuded, as shown in Figure 5.

Aspartic acid, phenylalanine, and methanol are the three readily available chemicals that constitute aspartame. It would seem a simple task to hook these molecules together, but such is not the case for there are several real challenges associated with any aspartame synthesis, as will be presented in the next section.
Aspartame Synthesis
As we discussed in the stereoisomerism heading of Section 1, certain organic compounds, because of their tetrahedral shape and atomic bonding arrangements, can exist in non-superimposable mirror image forms. (Think of your hands as non-superimposable mirror image objects.) Such isomers are called enantiomers and they have all their typical physical and chemical properties in common, but differ in the direction that they rotate plane polarized light and, more significantly, differ in the degree and/or manner in which they interact with other molecules that themselves can show such stereoisomeric character. This latter group contains biomolecules like enzymes and receptor sites like those that influence taste sensations.
Aspartame’s structure consists of two natural products (amino acids) linked together. Each of these natural materials can exist in their non-superimposable mirror images. In the natural world, however, only one of these isomers is commonly encountered. That form for aspartic acid is referred to as L-(+)-aspartic acid or S-(+)-aspartic acid (see Figure 6). The L (an older but commonly used symbol) and S labels indicate a specific mirror image (i.e., left or right hand) and the + indicates that this particular aspartic acid rotates plane polarized light in a clockwise direction.

Fig. 6
That aspartame was prepared initially by combining the two natural isomers is unsurprising since it is logically assumed that the naturally occurring amino acids are likely to be well tolerated in the body, whereas the unnatural isomers might not be.
If one wanted to prepare aspartame in ton quantities, it might be most cost effective if the aspartic acid and phenylalanine components could themselves be prepared in the lab from inexpensive precursors then linked together. When such syntheses are done in the lab, we usually get equal amounts of the R and S (D or L) isomers. Since neither R isomer is desired, it then becomes a challenge to separate the two isomers (since their properties are essentially identical) so that just the S isomer is available. Also, since half the products were the R isomers, this is not a very efficient procedure.
Phrased differently, the maximum yield of desired product is 50%, hardly a cost-effective procedure.
For the moment, let’s consider just combining both isomers of aspartic acid, both isomers of phenylalanine, and methanol together in hopes of preparing aspartame, S-aspartic acid-S-phenylalanine. What a mess! The R isomers are all unnecessary and unwanted, so when R-aspartic acid combined with S-phenylalanine or R-phenylalanine combined with S-aspartic acid, even if the combination occurred properly (as described later), an S-S isomer could not be formed. Of course, all R-R combinations would lead to an improper compound. The likelihood of combining an S isomer with an S isomer in the proper way is not very high. Clearly, the probability of preparing aspartame increases greatly if no R isomers are present in the reaction scheme. Pure S isomers are harder and more expensive to obtain, so cost will be added to the synthesis, but there is no good alternative.
But this hardly solves the synthetic problem. Let’s now consider mixing together just the S isomers of aspartic acid and phenylalanine along with methanol and analyzing the possibilities (see Figure 7). An S-phenylalanine molecule could condense its –NH2 with either –COOH of S-aspartic acid. The S-phenylalanine molecule could condense its –NH2 with a –COOH from another S-phenylalanine molecule. The S-aspartic acid might condense its correct –COOH with the –NH2 of S-phenylalanine, but it could also react with another of S-aspartic acid’s –NH2 using either –COOH. Also, the CH3OH could react with any of the three possible –COOH groups, not only the desired one. Even with the refinement effected by eliminating the presence of the R isomer, mixing the three reactants together is not a very satisfactory approach to the synthesis of aspartame. How do we overcome such hurdles? What strategies can be developed?

Clearly, one needs to work only with the desired two isomers. Synthetic or isolation techniques need to be developed so that the proper isomer can be available at a reasonable cost.
This has been done. Since the desired compounds used in the synthesis can condense in several different combinations, it makes sense to limit reaction choice so that the proper coupling can occur. This can be accomplished by creating a situation whereby no choice is possible except the one desired. This is frequently done by temporarily preventing or masking a group from reacting that otherwise might react, and later unmasking the group. This technique is called protection. This is not unlike handling a warm flask by first putting on a glove, moving the flask, and then removing the glove after the danger of burn is past. This is the strategy employed in the synthesis of aspartame.
Since the methanol needs to condense with phenylalanine’s –COOH, let’s do that before attempting to condense the phenylalanine with the aspartic acid to produce the methyl ester of phenylalanine. There are no competing processes and it also eliminates the possibility that the –COOH group in phenylalanine could condense with a –NH2 group of another phenylalanine or the –NH2 group in aspartic acid. This solves several potential problems in a convenient manner (Figure 8), but a couple of major ones still remain.
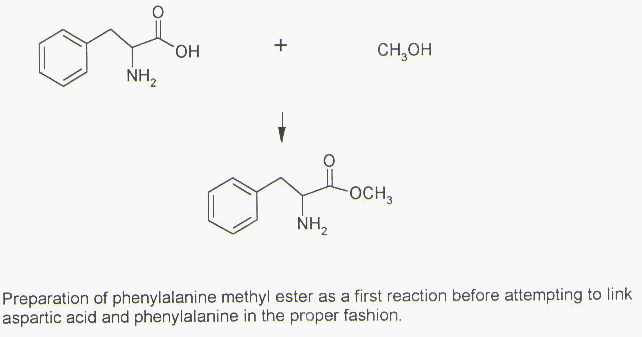
The challenge of condensing the –NH2 remaining on the S-phenylalanine methyl ester with the correct –COOH in S-aspartic acid is much more formidable. Several methods have been developed. A second challenge is to prevent aspartic acid from condensing with itself since it has both –NH2 and –COOH groups. The reaction complexity is beyond the scope of this ChemCase, but some of the ideas will be mentioned.
One approach has been to protect the –NH2 group in aspartic acid so that it cannot condense and then rendering the two –COOH groups sufficiently non-equivalent so that when the now protected aspartic acid reacts with the –NH2 of the methyl ester of phenylalanine, an aspartame-like molecule is obtained. The protective group is removed and, fortuitously, aspartame precipitates from solution leaving several impurities behind.
A clever and potentially excellent method of condensing the S-methyl ester of phenylalanine with S-aspartic acid could be to catalyze the reaction with an enzyme, as might occur in a natural system. A biocatalyst like an enzyme will likely differentiate between the two –COOH groups in aspartic acid and, if the right enzyme can be found, one could expect almost exclusive reaction at the desired –COOH. The enzyme catalyst might have difficulty differentiating between the –NH2 of phenylalanine, so protecting aspartic acid’s –NH2 from reacting might still be required. As before, the protecting group is removed after coupling. Indeed, many biocatalytic methods have been studied and successfully used to make aspartame.
Hopefully, you have gained some sense of the challenge associated with the synthesis of a relatively simple molecule such as aspartame from its three component parts. Not even mentioned in presenting the challenges are some undesired side reactions beyond those indicated that occur in most of the processes. As a consequence, better methods of preparing aspartame are being investigated to this day. The laboratory synthesis of aspartame is especially challenging for, in general, it is difficult to create situations where like groups are different. No, that’s not a misprint. Both aspartic acid and phenylalanine have –NH2 and –COOH groups, and to obtain aspartame we need to hook (condense) one particular –NH2 to one particular –COOH. We have to be able to differentiate between the similar –NH2 and –COOH groups. In our chemical synthesis, this has been done using protecting groups to ensure that only the correct –NH2 and the correct –COOH groups are available to react with each other. What would really be nice is if we could go back to our earlier chemical soup that contains S-aspartic acid, S-phenylalanine, and methanol and somehow put them together as desired to prepare aspartame. A natural biosystem might be able to accomplish such a task, but we are unaware of any natural system that produces aspartame. Some exploration of this concept has been undertaken, though preliminary results suggest that much refinement needs to occur. Solutions of S-aspartic acid and the methyl ester of S-phenylalanine have been incubated with strains of bacteria or yeast. It is reported that the yeast or bacteria synthesize small amounts of aspartame. Perhaps this technique can be exploited to prepare commercial quantities of aspartame in a cost-effective manner. Time will tell.
As you’ve waded through some of the complexities associated with the manufacture of aspartame, you can perhaps better appreciate the desire of corporations to protect their intellectual property and to recover the costs of research and development. These latter topics are taken up elsewhere in more detail.


