1C: NutraSweet: Stereoisomers
- Page ID
- 40233
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)We can learn most of the important principles of organic chemistry through a study of the aspartame molecule. In doing so, we will see the scientific problems that chemists faced as they attempted to synthesize and evaluate this artificial sweetener.
There is an additional subtlety regarding the aspartame structure that needs attention before we explore the interesting subject of sweeteners. A consequence of carbon’s sp3 tetrahedral shape and the attachment of four different groups or atoms to a tetrahedral carbon is a phenomenon called stereoisomerism. Isomers, in general, are defined as compounds with the same formula but different structures. The manner in which structures can differ can be substantial or can be of seemingly less consequence. The table below shows five isomer combinations and invites you to look at a three dimensional representation of two isomers of tartaric acid.
|
|
|
|
|
|
|
Pair 1 represents two different C6H12 compounds, one a cyclic saturated hydrocarbon and the other an unsaturated hydrocarbon. These isomers’ physical and chemical properties would differ, especially the chemical properties.
Two isomers for C2H6O (Pair 2) can be written. Their physical and chemical properties would also be quite different because they contain different functionalities.
The chloropropanes comprising Pair 3 have the same functionality, but the positions on the carbon chain are different. These compounds are chemically more similar than the isomeric pairs just discussed, so they show physical and chemical behavior that is similar.
The structural situation differs in Pairs 4 and 5 in that both have the same connectivity within their respective isomer pairs. They both have the same atoms bonded to the same atoms in each isomer, but the spaciality of the atoms (groups of atoms) differs in each. The spatial difference is a consequence of carbon’s hybridization and, in the case of Pair 4, restricted rotation. Isomers with the same connectivity but different structures (which is what makes them isomers) are called stereoisomers.
One aspect of overall shape not yet mentioned relates to "bond rotation," the ability of atoms to move relative to each other in space about a single bond. In Figure 8, the central C-C bond in butane is exaggerated to compare it with the central C=C bond in butene-2.

Under normal conditions of temperature, there is sufficient energy to effect rotation about any single C-C, C-O, C-H, or C-N bond. The preferred three-dimensional molecular shape, the so-called conformational shape, will be determined by overall energy. For butane, it makes energetic sense for the molecule to assume a conformation that places the CH3 groups away from each other, so that they do not get in each other’s space and interfere sterically (the term we use to describe such crowding). With the 2-butene, however, rotation is restricted because any rotation about the C=C would necessarily break the pi bond. Consequently, the four attached groups are restricted to the positions shown and two isomers are possible here. The cis isomer has the two CH3 groups on the same side; the trans isomers have them on opposite sides. These two isomers are similar, but will have different boiling points, densities, etc. Such isomers are called diastereoisomers or, more specifically, geometric isomers.
Which isomer, cis or trans butene, would you expect to be energetically more favorable?
Why?
The components of Pair V, like those of Pair IV, have the same connectivity but are not superimposable on each other, a criterion for being the same compound. The relationship between the two isomers is that of non-superimposable mirror images (think right and left hands). Such isomers are a consequence of the four different attachments to the tetrahedral sp3 carbon. Such a carbon is called a chiral (or asymmetric) center. The first instance of discovery of such isomers is attributed to Pasteur who serendipitously observed mirror image crystals formed from tartaric acid salts derived from the wine industry.
It would be very difficult to reproduce the experimental conditions that led to his discovery, for it is extraordinarily uncommon to be able to observe such differences as crystal structure in isomers of this sort. Non-superimposable mirror images are called enantiomers. The two tartaric acid enantiomers are shown as Pair V. What makes Pasteur’s discovery so special (though someone would have eventually made it) is that it occurred before our modern understanding of bonding theory and molecular shapes and because the physical and chemical properties of the two tartaric acid isomers are identical. They have the same melting points, the same solubilities, and the same chemical reactions at the same rates (except when reacting with other chiral compounds). For identification purposes, the one property in which they differ is the direction in which they rotate plane polarized light. One isomer plane polarized light clockwise while the other enantiomer plane polarized the light counterclockwise.
Example
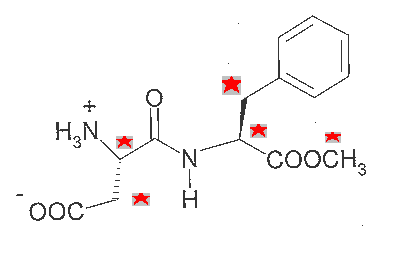
Nutrasweet is made from two amino acids, phenylalanine and aspartic acid. Each of these has a chiral center. From the structure, can you identify the chiral carbon atom in each?
Image of phenylalanine
Image of aspartic acid
(You may need to Download RasMol to view these structures. Click on .pdb on the cited pages to look at 3D structures through Rasmol, click on .gif to view planar images through your browser.)
To distinguish isomers without drawing the structures, terminology is used in the naming of compounds (nomenclature) to describe stereoisomers. For the geometric isomers, cis (or Z) is used to indicate like groups, in our example the CH3 or H groups, on the same side of the C=C. Trans (E) indicates the like groups on opposite sides. For enantiomers, modern nomenclature uses R and S designations, but older literature uses D and L designations for amino acids (and sugars), which carry over to current use. We will not study the rules that allow one to use these symbols.
The importance of the concept of stereoisomers, particularly enantiomers, is dramatically evident in most biological processes that occur in the natural world, including something as routine and as pleasing as taste sensation. This subject will be explored in the next section.
Example
Find the chiral centers in NutraSweet. Which of the five sp3 carbon atoms are chiral (have four different groups attached)?